
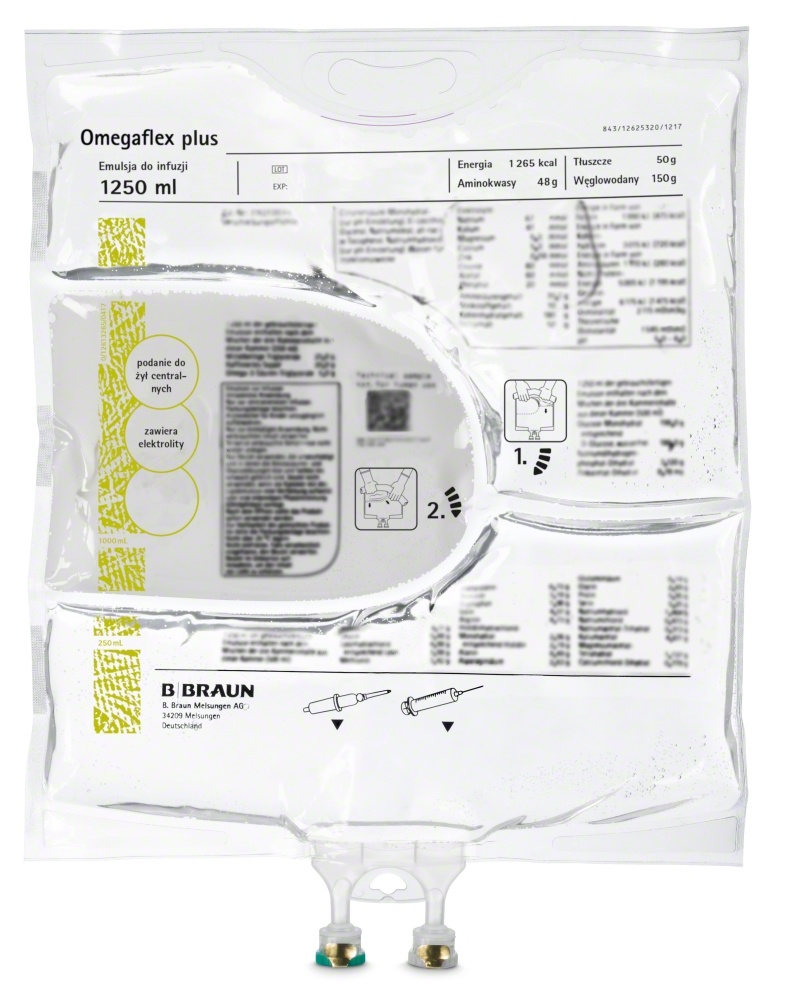
Omegaflex plus

Ask a doctor about a prescription for Omegaflex plus

How to use Omegaflex plus
Leaflet accompanying the packaging: Information for the user
Omegaflex plus
Infusion emulsion
Please read carefully the contents of the leaflet before using the medicine, as it contains
important information for the patient.
- Please keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Omegaflex plus and what is it used for
- 2. Important information before using Omegaflex plus
- 3. How to use Omegaflex plus
- 4. Possible side effects
- 5. How to store Omegaflex plus
- 6. Contents of the packaging and other information
1. What is Omegaflex plus and what is it used for
Omegaflex plus contains fluids and substances called amino acids, electrolytes, and fatty acids, which are necessary for the growth of the body or for recovery. It also contains calories in the form of carbohydrates and fats.
Omegaflex plus is indicated for use in adults, adolescents, and children over two years of age.
The patient receives Omegaflex plus when they cannot normally take food. This can happen in many situations, for example, when the patient is recovering from surgery, injuries, or burns, or when they cannot absorb food from the stomach and intestines.
2. Important information before using Omegaflex plus
When not to use Omegaflex plus
- if the patient is allergic to any active substance, eggs, peanuts, soy, or fish, or any of the excipients of this medicine (listed in section 6);
- this medicine must not be administered to newborns, infants, and young children under two years of age.
Omegaflex plus must not be used if the patient has any of the following conditions:
- life-threatening circulatory disorders, such as those occurring in shock or collapse;
- heart attack or stroke;
- severe blood clotting disorders, risk of bleeding (severe coagulopathy, worsening hemorrhagic diathesis);
- blockage of blood vessels by blood clots or fat (embolism);
- severe liver failure;
- disorders of bile flow (intrahepatic cholestasis);
- severe kidney failure in the absence of renal replacement therapy;
- electrolyte balance disorders;
- fluid deficiency or excess water in the body;
- fluid in the lungs (pulmonary edema);
- severe heart failure;
- certain metabolic disorders, such as:
- excessive amounts of lipids (fats) in the blood,
- inborn errors of amino acid metabolism,
- abnormally high blood sugar levels requiring more than 6 units of insulin per hour,
- metabolic disorders that may occur after surgical procedures or injuries,
- coma of unknown origin,
- inadequate tissue oxygen supply,
- abnormally high levels of acidic substances in the blood.
Warnings and precautions
Before starting treatment with Omegaflex plus, discuss it with your doctor.
Please inform your doctor if:
- the patient has heart, liver, or kidney problems;
- the patient has certain metabolic disorders, such as diabetes, abnormal blood fat levels, and disorders of fluid and electrolyte balance or acid-base balance.
During treatment with this medicine, the patient will be closely monitored to detect early signs of an allergic reaction (such as fever, chills, rash, or shortness of breath).
To ensure that the patient's body is properly processing the administered nutrients, further observations and tests will be performed, such as various blood sample tests.
The medical staff will also take measures to ensure that the patient's fluid and electrolyte needs are met. In addition to Omegaflex plus, the patient will also receive other nutrients to fully meet their body's needs.
Children
This medicine must not be administered to newborns, infants, and young children under two years of age.
Omegaflex plus and other medicines
Tell your doctor about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
Omegaflex plus may interact with some other medicines. Tell your doctor if the patient is taking or receiving any of the following medicines:
- insulin;
- heparin;
- medicines that prevent unwanted blood clotting, such as warfarin or other coumarin derivatives;
- medicines that improve urine production (diuretics);
- medicines used to treat high blood pressure or heart disease (ACE inhibitors and angiotensin II receptor antagonists);
- medicines used in organ transplantation, such as cyclosporine and tacrolimus;
- medicines used to treat inflammatory conditions (corticosteroids);
- hormonal preparations that affect fluid balance (adrenocorticotropic hormone [ACTH]).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine. If the patient is pregnant, they will receive this medicine only if the doctor considers it absolutely necessary for their recovery. There is no data on the use of Omegaflex plus in pregnant women.
Breastfeeding is not recommended for mothers receiving parenteral nutrition.
Driving and using machines
This medicine is usually administered to bedridden patients, e.g., in a hospital or clinic, which excludes driving and using machines. However, the medicine itself does not affect the ability to drive and use machines.
Omegaflex plus contains sodium
The medicine contains 0.931 mg/ml of sodium (the main component of table salt). This corresponds to 0.047% of the maximum recommended daily intake of sodium in the diet for adults.
The maximum recommended daily dose of this medicinal product contains 2607 mg of sodium (contained in table salt). This corresponds to 130% of the maximum recommended daily intake of sodium in the diet for adults.
In case of administration of one or more bags per day for a longer period, patients, especially those controlling their sodium intake, should consult their doctor or pharmacist.
3. How to use Omegaflex plus
This medicine is administered by intravenous infusion (drip), i.e., through a thin tube directly into a vein. This medicine will be administered exclusively through one of the large (central) veins. The recommended infusion time for a single bag of emulsion for parenteral nutrition is a maximum of 24 hours.
The doctor will decide how much of this medicine the patient needs and how long they will require treatment with this medicine.
Use in children
This medicine must not be administered to newborns, infants, and young children under two years of age.
The doctor will decide how much of this medicine the child needs and how long they will require treatment with this medicine.
Using more than the recommended dose of Omegaflex plus
In case of administration of too much of this medicine, the patient may experience an overload syndrome and the following symptoms may occur:
- excess fluid and electrolyte disturbances;
- fluid in the lungs (pulmonary edema);
- loss of amino acids in the urine and amino acid balance disorders;
- nausea, vomiting;
- chills;
- high blood sugar levels;
- glucose in the urine;
- fluid deficiency;
- significantly higher levels of blood components than normal (hyperosmolality);
- disorders or loss of consciousness due to extremely high blood sugar levels;
- enlargement of the liver (hepatomegaly) with jaundice or without;
- enlargement of the spleen (splenomegaly);
- fat deposition in internal organs;
- abnormal liver function test results;
- decreased red blood cell count (anemia);
- decreased white blood cell count (leukopenia);
- decreased platelet count (thrombocytopenia);
- increased number of immature red blood cells (reticulocytosis);
- breakdown of red blood cells (hemolysis);
- bleeding or tendency to bleed;
- blood clotting disorders (which can be determined by changes in bleeding time, clotting time, prothrombin time, etc.);
- fever;
- high levels of fats in the blood;
- loss of consciousness.
In case of any of these symptoms, the infusion should be stopped immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may be serious. If you experience any of these side effects, tell your doctor immediately, who will stop the administration of the medicine:
Rare (may affect up to 1 in 1000 people)
- allergic reactions, such as skin reactions, shortness of breath, swelling of the lips, mouth, and throat, respiratory disorders.
Other side effects:
Uncommon (may affect up to 1 in 100 people):
- nausea, vomiting, loss of appetite.
Rare (may affect up to 1 in 1000 people):
- increased tendency to blood clotting,
- blue discoloration of the skin,
- shortness of breath,
- headache,
- hot flashes,
- redness of the skin (flushing),
- sweating,
- chills,
- feeling cold,
- high body temperature,
- drowsiness,
- chest pain, back pain, bone pain, or lumbar pain,
- decreased or increased blood pressure.
Very rare (may affect up to 1 in 10,000 people):
- abnormally high levels of fat or sugar in the blood,
- high levels of acidic substances in the blood,
- excessive amounts of fat may lead to a fat overload syndrome. More information is provided in section 3 "Using more than the recommended dose of Omegaflex plus". Symptoms usually resolve after stopping the infusion.
Frequency not known (cannot be estimated from the available data):
- decreased white blood cell count (leukopenia),
- decreased platelet count (thrombocytopenia),
- disorders of bile flow (cholestasis).
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C,
02-222 Warsaw,
tel.: 22 49-21-301, fax: 22 49-21-309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Omegaflex plus
Store the medicine out of sight and reach of children.
Do not store above 25°C.
Do not freeze. In case of accidental freezing, discard the bag.
Do not use this medicine after the expiry date stated on the label after EXP. The expiry date refers to the last day of the month.
Store the bag in the outer protective bag to protect it from light.
6. Contents of the packaging and other information
What Omegaflex plus contains
The active substances of this ready-to-use mixture are:
| from the upper chamber (glucose solution) | in 1000 ml | in 1250 ml | in 1875 ml | in 2500 ml |
| Glucose monohydrate which corresponds to glucose | 132.0 g 120.0 g | 165.0 g 150.0 g | 247.5 g 225.0 g | 330.0 g 300.0 g |
| Sodium diphosphate dihydrate | 1.872 g | 2.340 g | 3.510 g | 4.680 g |
| Zinc acetate dihydrate | 5.264 mg | 6.580 mg | 9.870 mg | 13.16 mg |
| from the middle chamber (fat emulsion) | in 1000 ml | in 1250 ml | in 1875 ml | in 2500 ml |
| Triglycerides of saturated fatty acids with medium chain length | 20.00 g | 25.00 g | 37.50 g | 50.00 g |
| Purified soybean oil | 16.00 g | 20.00 g | 30.00 g | 40.00 g |
| Omega-3 fatty acid triglycerides | 4.000 g | 5.000 g | 7.500 g | 10.00 g |
| from the lower chamber (amino acid solution) | in 1000 ml | in 1250 ml | in 1875 ml | in 2500 ml |
| Isoleucine | 2.256 g | 2.820 g | 4.230 g | 5.640 g |
| Leucine | 3.008 g | 3.760 g | 5.640 g | 7.520 g |
| Lysine hydrochloride which corresponds to lysine content | 2.728 g 2.184 g | 3.410 g 2.729 g | 5.115 g 4.094 g | 6.820 g 5.459 g |
| Methionine | 1.880 g | 2.350 g | 3.525 g | 4.700 g |
| Phenylalanine | 3.368 g | 4.210 g | 6.315 g | 8.420 g |
| Threonine | 1.744 g | 2.180 g | 3.270 g | 4.360 g |
| Tryptophan | 0.544 g | 0.680 g | 1.020 g | 1.360 g |
| Valine | 2.496 g | 3.120 g | 4.680 g | 6.240 g |
| Arginine | 2.592 g | 3.240 g | 4.860 g | 6.480 g |
| Histidine hydrochloride monohydrate which corresponds to histidine content | 1.624 g 1.202 g | 2.030 g 1.503 g | 3.045 g 2.254 g | 4.060 g 3.005 g |
| Alanine | 4.656 g | 5.820 g | 8.730 g | 11.64 g |
| Aspartic acid | 1.440 g | 1.800 g | 2.700 g | 3.600 g |
| Glutamic acid | 3.368 g | 4.210 g | 6.315 g | 8.420 g |
| Glycine | 1.584 g | 1.980 g | 2.970 g | 3.960 g |
| Proline | 3.264 g | 4.080 g | 6.120 g | 8.160 g |
| Serine | 2.880 g | 3.600 g | 5.400 g | 7.200 g |
| Sodium hydroxide | 0.781 g | 0.976 g | 1.464 g | 1.952 g |
| Sodium chloride | 0.402 g | 0.503 g | 0.755 g | 1.006 g |
| Sodium acetate trihydrate | 0.222 g | 0.277 g | 0.416 g | 0.554 g |
| Potassium acetate | 2.747 g | 3.434 g | 5.151 g | 6.868 g |
| Magnesium acetate tetrahydrate | 0.686 g | 0.858 g | 1.287 g | 1.716 g |
| Calcium chloride dihydrate | 0.470 g | 0.588 g | 0.882 g | 1.176 g |
Other ingredients are citric acid monohydrate (to adjust pH), egg lecithin for injection, glycerol, sodium oleate, all-rac-α-tocopherol, sodium hydroxide (to adjust pH), and water for injection.
What Omegaflex plus looks like and what the packaging contains
The ready-to-use product is an infusion emulsion, i.e., it is an emulsion administered through a thin tube into a vein.
Omegaflex plus is supplied in flexible three-chamber bags containing:
- 1250 ml (500 ml amino acid solution + 250 ml fat emulsion + 500 ml glucose solution)
| in 1000 ml | in 1250 ml | in 1875 ml | in 2500 ml | |
| Amino acid content [g] | 38 | 48 | 72 | 96 |
| Nitrogen content [g] | 5.4 | 6.8 | 10.2 | 13.7 |
| Carbohydrate content [g] | 120 | 150 | 225 | 300 |
| Fat content [g] | 40 | 50 | 75 | 100 |
| Electrolytes [mmol] | in 1000 ml | in 1250 ml | in 1875 ml | in 2500 ml |
| Sodium | 40 | 50 | 75 | 100 |
| Potassium | 28 | 35 | 52.5 | 70 |
| Magnesium | 3.2 | 4.0 | 6.0 | 8.0 |
| Calcium | 3.2 | 4.0 | 6.0 | 8.0 |
| Zinc | 0.024 | 0.03 | 0.045 | 0.06 |
| Chloride | 36 | 45 | 67.5 | 90 |
| Acetate | 36 | 45 | 67.5 | 90 |
| Phosphate | 12 | 15 | 22.5 | 30 |
| in 1000 ml | in 1250 ml | in 1875 ml | in 2500 ml | |
| Energy from fat [kJ (kcal)] | 1590 (380) | 1990 (475) | 2985 (715) | 3980 (950) |
| Energy from carbohydrates [kJ (kcal)] | 2010 (480) | 2510 (600) | 3765 (900) | 5020 (1200) |
| Energy from amino acids [kJ (kcal)] | 635 (150) | 800 (190) | 1200 (285) | 1600 (380) |
| Non-protein energy [kJ (kcal)] | 3600 (860) | 4500 (1075) | 6750 (1615) | 9000 (2155) |
| Total energy [kJ (kcal)] | 4235 (1010) | 5300 (1265) | 7950 (1900) | 10600 (2530) |
| Osmolality [mOsm/kg] | 1540 |
| Theoretical osmolality [mOsm/l] | 1215 |
| pH | 5.0-6.0 |
- 1875 ml (750 ml amino acid solution + 375 ml fat emulsion + 750 ml glucose solution)
- 2500 ml (1000 ml amino acid solution + 500 ml fat emulsion + 1000 ml glucose solution)
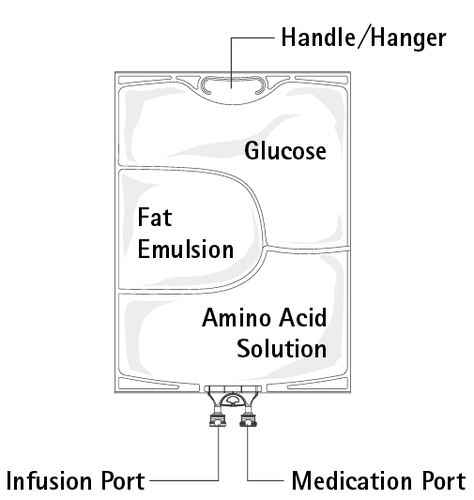
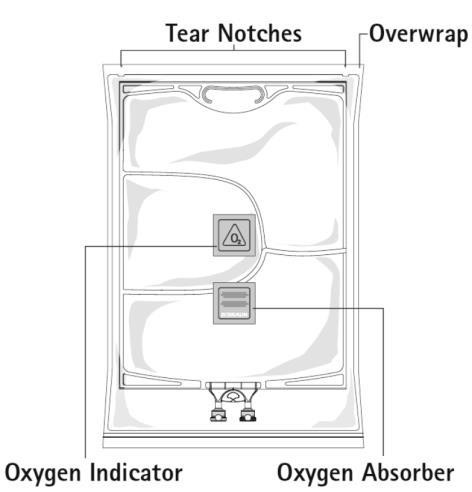
Figure A
Figure B
Figure A: The multi-chamber bag is packaged in an outer protective bag. Between the inner and outer bags, there is an oxygen absorber and an oxygen indicator. The oxygen absorber sachet is made of neutral material and contains iron hydroxide.
Figure B: The upper chamber contains glucose solution, the middle chamber contains fat emulsion, and the lower chamber contains amino acid solution.
The glucose and amino acid solutions are clear, colorless to straw-colored. The fat emulsion is milky white.
The upper and middle chambers can be connected to the lower chamber by opening the internal welds.
Different package sizes are supplied in cardboard boxes containing five bags.
Package sizes: 5 x 1250 ml, 5 x 1875 ml, and 5 x 2500 ml
Not all package sizes may be marketed.
Marketing authorization holder
- B. Braun Melsungen AG Carl-Braun-Straße 1 34212 Melsungen, Germany
Tel.: +49-5661-71-0
Fax: +49-5661-71-4567
Manufacturer
- B. Braun Melsungen AG Carl-Braun-Straße 1 34212 Melsungen, Germany
To obtain more detailed information on this medicine, please contact the representative of the marketing authorization holder in Poland:
Aesculap Chifa sp. z o.o.
ul. Tysiąclecia 14
64-300 Nowy Tomyśl
Phone: +48 61 442 01 00
Fax: +48 61 443 75 05
Email: [email protected]
This medicine is authorized in the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria
Nutriflex Omega plus B.Braun Emulsion zur Infusion
Belgium
Nutriflex Omega plus 38 g/l Amino + 120 g/l G, emulsie voor infusie
Bulgaria
Nutriflex Omega 38/120 emulsion for infusion
Croatia
Nutriflex Omega 38/120 plus emulzija infuziju
Czech Republic
Nutriflex Omega plus 38/120
Denmark
Nutriflex Omega Plus
Finland
Nutriflex Omega 38/120/40 infuusioneste, emulsio
France
MEDNUTRIFLEX OMEGA E, émulsion pour perfusion
Germany
NuTRIflex Omega plus novo Emulsion zur Infusion
Greece
Nutriflex Omega 38/120 plus
Ireland
Omeflex plus emulsion for infusion
Italy
Omegaflex AA38/G120 emulsione per infusione
Luxembourg
NuTRIflex Omega plus novo Emulsion zur Infusion
Netherlands
Nutriflex Omega plus 38 g/l Amino + 120 g/l G, emulsie voor infusie
Norway
Nutriflex Omega Plus infusjonsvæske, emulsjon
Poland
Omegaflex plus
Portugal
Nutriflex Omega 38/120 P emulsão para perfusão
Romania
NuTRIflex Omega Plus novo emulsie perfuzabilă
Slovakia
Nutriflex Omega plus 38/120
Spain
Nutriflex Omega 38/120 plus emulsión para perfusión
Sweden
Nutriflex Omega 38/120/40 infusionsvätska, emulsion
United Kingdom (Northern Ireland)
Omeflex plus emulsion for infusion
Date of last revision of the leaflet: 2024-01-19 __________________________________________________________________________
Information intended for healthcare professionals only:
Before use, parenteral nutrition products should be visually inspected for any signs of damage, color change, and emulsion instability.
Do not use damaged bags. The outer and inner bags, as well as the welds between the chambers, should be intact. The product should only be used if the amino acid and glucose solutions are clear and colorless to straw-colored, and the fat emulsion is homogeneous in a milky white color. Do not use if the solutions contain solid particles.
After mixing the contents of the three chambers, do not use if the emulsion shows discoloration or signs of phase separation (oil droplets, oil layer). In case of emulsion discoloration or signs of phase separation, stop the infusion immediately.
Before opening the outer bag, check the color of the oxygen indicator (see Figure A). Do not use if the oxygen indicator has changed color to pink. Use only if the oxygen indicator is yellow.
Preparing the mixed emulsion
Strictly follow aseptic procedures.
Opening: Tear the outer bag, starting from the cuts (Fig. 1). Remove the inner bag from the protective packaging. Discard the outer packaging, oxygen indicator, and oxygen absorber.
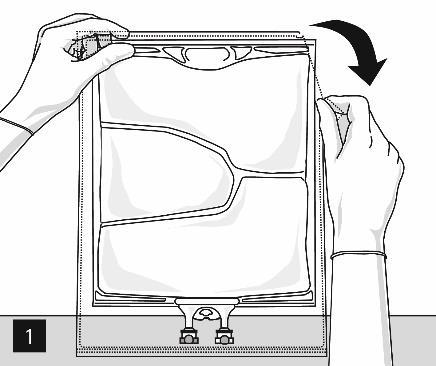
Visually inspect the inner bag for any signs of leakage.
A leaking bag should be discarded, as sterility cannot be guaranteed.
Mixing the bag contents and adding additional substances
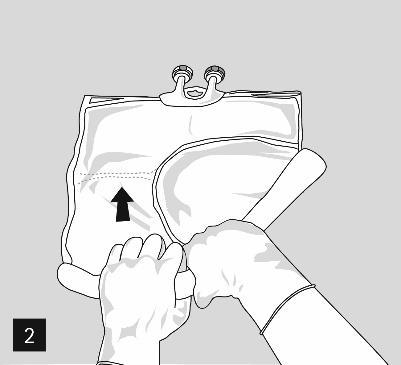
To sequentially open the chambers and mix their contents, fold the bag with both hands, starting from the weld separating the upper chamber (glucose) and the lower chamber (amino acids) (Fig. 2).
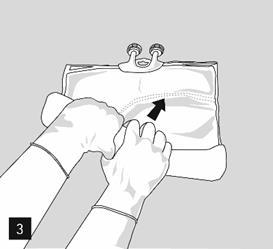
Then, continue to press to open the weld separating the middle chamber (fat) and the lower chamber (Fig. 3).
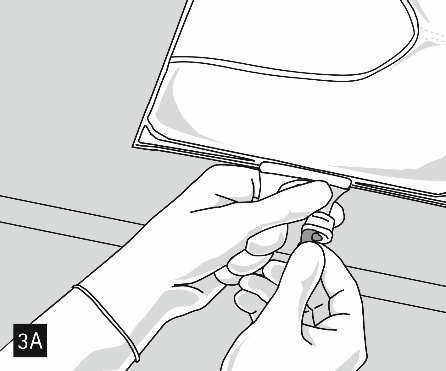
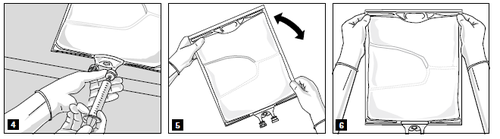
After mixing the contents of all chambers and removing the aluminum foil (Fig. 3A), compatible additives can be added through the drug port (Fig. 4). Thoroughly mix the contents (Fig. 5) and visually inspect the mixture (Fig. 6). The mixture is a milky white homogeneous emulsion of the oil-in-water type. Any signs of phase separation of the emulsion are not acceptable.
- 6) and visually inspect the mixture (Fig. 6). The mixture is a milky white homogeneous emulsion of the oil-in-water type. Any signs of phase separation of the emulsion are not acceptable.
Omegaflex plus can be mixed with the following additives up to the specified upper limits of concentration or maximum amount of additives after supplementation. The resulting mixtures are stable for 7 days at a temperature of +2°C to +8°C plus 2 days at 25°C.
- Electrolytes: consider the electrolytes already present in the bag; in the three-component mixture, stability has been demonstrated up to a total of 200 mmol/l of sodium + potassium (together), 9.6 mmol/l of magnesium, and 6.4 mmol/l of calcium.
- Phosphate: stability has been demonstrated up to a maximum concentration of 20 mmol/l of inorganic phosphate.
- Alanyl-glutamine up to 24 g/l.
- Trace elements and vitamins: stability has been demonstrated using commercially available products containing a large number of trace elements and vitamins (e.g., Tracutil, Cernevit) up to the standard dose recommended by the respective manufacturer of the micronutrient preparation.
Detailed information on the above-mentioned additives and the corresponding shelf-life of such mixtures can be obtained on request from the manufacturer.
Preparation for infusion
Before infusion, the emulsion should be warmed to room temperature.
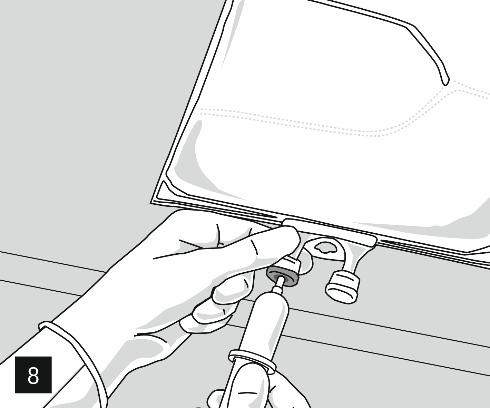
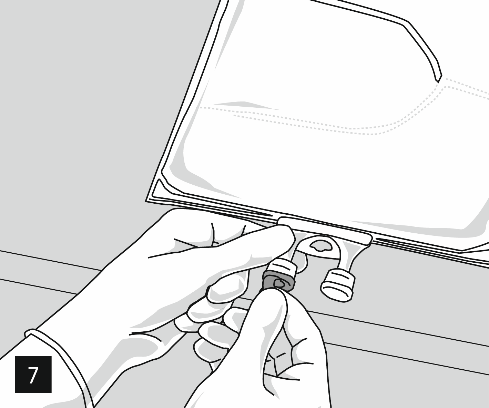
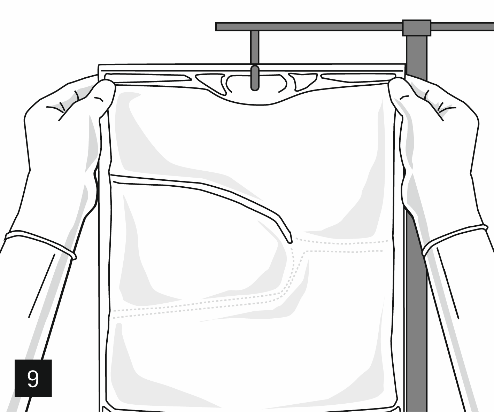
Remove the aluminum cap from the infusion port (Fig. 7) and connect the infusion set (Fig. 8). Use an infusion set without an air vent or cover the air vent when using a set with an air vent. Hang the bag on the infusion stand (Fig. 9) and perform the infusion using standard technique.
For single use only. After use, discard the packaging and any unused product.
Any unused product or waste material should be disposed of in accordance with local regulations.
Do not reconnect partially used containers.
If filters are used, they must be fat-permeable (pore size ≥ 1.2 µm).
Shelf-life after opening the outer bag and after mixing the bag contents
Chemical and physicochemical stability of the mixed amino acid, glucose, and fat mixture has been demonstrated before use for a period of 7 days at a temperature of 2-8°C and for an additional 2 days at 25°C.
Shelf-life after adding compatible additives
From a microbiological point of view, the product should be used immediately after adding additives. If the product is not used immediately after adding additives, the responsibility for the storage period and storage conditions before use lies with the user.
After the first opening (puncture of the infusion port)
The emulsion should be used immediately after opening the packaging.
Omegaflex plus should not be mixed with other medicinal products for which compatibility has not been demonstrated.
Due to the risk of pseudoagglutination of Omegaflex plus, it should not be administered simultaneously with blood through the same infusion set.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterB. Braun Melsungen AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Omegaflex plusDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not required
Alternatives to Omegaflex plus in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Omegaflex plus in Іспанія
Alternative to Omegaflex plus in Україна
Online doctors for Omegaflex plus
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Omegaflex plus – subject to medical assessment and local rules.










