
Olimel N12e

Ask a doctor about a prescription for Olimel N12e

How to use Olimel N12e
Patient Information Leaflet: User Information
OLIMEL N12E, infusion emulsion
Read the leaflet carefully before administering the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should inform their doctor or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What is OLIMEL N12E, infusion emulsion and what is it used for
- 2. Important information before administering OLIMEL N12E, infusion emulsion
- 3. How to use OLIMEL N12E, infusion emulsion
- 4. Possible side effects
- 5. How to store OLIMEL N12E, infusion emulsion
- 6. Contents of the pack and other information
1. What is OLIMEL N12E, infusion emulsion and what is it used for
OLIMEL N12E is an infusion emulsion. The medicine is supplied in a triple-chamber bag.
The first chamber contains a glucose solution with calcium, the second chamber contains a fat emulsion, and the third chamber contains an amino acid solution with other electrolytes.
OLIMEL N12E is used for intravenous nutrition in adults and children over 2 years of age when oral nutrition is not appropriate.
OLIMEL N12E should only be used under medical supervision.
2. Important information before administering OLIMEL N12E, infusion emulsion
When not to use OLIMEL N12E, infusion emulsion:
- in premature infants, newborns, and children under 2 years of age;
- if the patient is allergic to eggs, soy, peanut proteins, corn/corn products (see also "Warnings and precautions" below) or any of the other ingredients of this medicine (listed in section 6);
- if the use of certain amino acids causes an abnormal reaction in the patient's body;
- if the patient has a particularly high level of fats in the blood;
- if the patient has hyperglycemia (too high blood sugar levels);
- if the patient's blood has an abnormally high content of any of the electrolytes (sodium, potassium, magnesium, calcium, and/or phosphorus).
In each case, the doctor will decide whether to administer the medicine based on factors such as the patient's age, body weight, and health status, including the results of any tests performed.
Warnings and precautions
Before starting the administration of OLIMEL N12E, discuss it with your doctor or nurse.
Too rapid administration of total parenteral nutrition solutions may result in injury or death of the patient.
If unusual signs or symptoms of an allergic reaction occur (such as sweating, fever, chills, headache, skin rash, or breathing difficulties), the infusion should be stopped immediately. The medicine contains soybean oil and egg phospholipids. Soy and egg proteins may cause hypersensitivity reactions. Cross-allergic reactions have been observed between soy and peanut proteins.
OLIMEL N12E contains glucose derived from corn, which may cause hypersensitivity reactions if the patient is allergic to corn or corn products (see "When not to use OLIMEL N12E, infusion emulsion" above).
Breathing difficulties may also be a sign that small particles are blocking blood vessels in the lungs (pulmonary embolism). If breathing difficulties occur, the doctor or nurse should be informed. They will decide on the appropriate action.
The antibiotic ceftriaxone should not be mixed or administered simultaneously with any calcium-containing solutions (including OLIMEL N12E) by intravenous infusion.
These medicines should not be administered simultaneously even through different infusion lines or injection sites.
However, OLIMEL N12E and ceftriaxone can be administered sequentially, one after the other, if the infusion lines are inserted at different sites or are replaced or thoroughly flushed with physiological saline solution between infusions to avoid precipitate formation.
Certain medicines and diseases may increase the risk of infection or sepsis (presence of bacteria in the blood). There is a particular risk of infection or sepsis after inserting a catheter (central venous catheter) into the patient's vein. The doctor will closely monitor the patient to detect any signs of infection. Patients requiring parenteral nutrition (administration of nutrients through a vein) are, due to their health status, more prone to developing infections. Following aseptic procedures during catheter placement, handling, and preparation of the nutrition solution (total parenteral nutrition) can reduce the risk of infection.
If the patient is severely malnourished and needs to receive food through a vein, the doctor should start treatment slowly. At the same time, the doctor should closely monitor the patient to prevent sudden changes in fluid, vitamin, electrolyte, and mineral levels.
Before starting the infusion, the patient's water and electrolyte balance and metabolic disorders should be corrected. The doctor will monitor the patient during therapy and may adjust the dosage or prescribe additional nutritional supplements, such as vitamins, electrolytes, and trace elements, if necessary.
In patients receiving intravenous nutrition, liver function disorders have been reported, including difficulties in removing bile (cholestasis), fat accumulation (fatty liver), fibrosis, possibly leading to liver failure, as well as gallbladder inflammation and gallstones. The causes of these disorders are thought to be different in different patients. If the patient experiences symptoms such as nausea, vomiting, abdominal pain, yellowing of the skin or eyes, they should consult their doctor to identify possible causes and therapeutic measures.
The doctor should be informed about:
- severe kidney disease. The doctor should also be informed if the patient is undergoing dialysis (artificial kidney) or other blood purification methods;
- severe liver disease;
- blood coagulation disorders;
- adrenal insufficiency (adrenal gland failure). The adrenal glands are triangular glands located on top of the kidneys;
- heart failure;
- lung disease;
- fluid accumulation in the body (overhydration);
- insufficient fluid in the body (dehydration);
- untreated high blood sugar levels (diabetes);
- heart attack or shock caused by sudden heart failure;
- severe metabolic acidosis (too acidic blood);
- sepsis (blood infection);
- coma.
To check the effectiveness and safety of the medicine, the patient will undergo clinical and laboratory tests ordered by the doctor during treatment. If the medicine is administered for several weeks, the patient's blood will be regularly tested.
A decreased ability of the body to remove lipids contained in the administered medicine may result in a condition called fat overload syndrome (see section 4 - "Possible side effects").
If pain, burning, or swelling occurs at the infusion site or if the infused fluid leaks during infusion, the doctor or nurse should be informed.
The administration of the medicine will be stopped immediately and then resumed in a different vein.
If blood sugar levels increase excessively, the doctor should adjust the rate of administration of OLIMEL N12E or administer a medicine to regulate blood sugar levels (insulin).
OLIMEL N12E can be administered through a catheter (central venous catheter) inserted into a large vein in the patient's chest.
Children and adolescents
When used in children under 18 years of age, particular caution should be exercised to administer the correct dose of the medicine. Due to the increased susceptibility of children to the risk of infection, increased precautions should also be taken. Vitamin and trace element supplementation is always required. For children, pediatric compositions and quantities must be used.
OLIMEL N12E and other medicines
Tell your doctor about all medicines you are taking or using now or recently, and about medicines you plan to take or use.
Concomitant use of other medicines is usually not contraindicated. However, you should inform your doctor about all medicines taken recently, including those available without a prescription, to check their compatibility.
Inform your doctor about taking or receiving:
- insulin,
- heparin.
Do not administer OLIMEL N12E simultaneously with blood through the same infusion set.
OLIMEL N12E contains calcium. It should not be administered with the antibiotic ceftriaxone or through the same infusion line, as particles may form. If these medicines are administered sequentially using the same device, it should be thoroughly flushed.
Due to the risk of precipitate formation, OLIMEL N12E should not be administered through the same infusion line or added to the antibiotic ampicillin or the antiepileptic medicine fosphenytoin.
The olive and soybean oil present in OLIMEL N12E contain vitamin K. This usually has no effect on the action of blood-thinning medicines (anticoagulants) such as coumarin. However, if you are taking anticoagulant medicines, you should inform your doctor.
The fats in the emulsion may interfere with the results of certain laboratory tests if the blood sample for testing is taken before the fats are removed from the patient's bloodstream (they are removed from the blood after 5 to 6 hours after fat administration).
OLIMEL N12E contains potassium. Particular caution should be exercised in patients taking diuretic medicines, ACE inhibitors, angiotensin II receptor antagonists (medicines used in hypertension), or immunosuppressive medicines. These types of medicines may cause an increase in potassium levels in the blood.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor for advice before taking this medicine.
There is limited experience with the use of OLIMEL N12E in pregnant or breastfeeding women. If necessary, OLIMEL N12E may be considered during pregnancy and breastfeeding. OLIMEL N12E should be used in pregnant or breastfeeding women only after careful consideration.
Fertility
No relevant data are available.
Driving and using machines
Not applicable.
3. How to use OLIMEL N12E, infusion emulsion
Dosage
OLIMEL N12E should only be used in adults and children over 2 years of age.
The medicine is an infusion emulsion administered through a catheter (central venous catheter) into a large vein in the patient's chest.
Before administration, OLIMEL N12E should be at room temperature.
OLIMEL N12E is for single use only.
The infusion of one bag usually lasts from 12 to 24 hours.
Adult dosage
The rate of administration, according to the patient's needs and clinical condition, will be determined by the doctor.
The medicine can be used for as long as necessary, depending on the patient's clinical condition.
Children over 2 years of age and adolescents
The dose of the medicine and the duration of administration are determined by the doctor. This depends on the patient's age, body weight, height, health status, and ability to break down and utilize the components of OLIMEL N12E.
Administration of a higher dose of OLIMEL N12E, infusion emulsion than recommended
If the patient receives too high a dose of the medicine or too rapid an infusion, the contained amino acids may contribute to an increase in blood acidity and the occurrence of symptoms of hypervolemia (increased blood volume). Blood sugar levels and urine sugar levels may increase, and a condition called hyperosmolar syndrome (excessive blood viscosity) may occur, and the fats in the emulsion may increase triglyceride levels in the blood. Administration of the medicine too quickly or in too large a volume may cause nausea, vomiting, chills, headache, flushing, excessive sweating, and electrolyte disturbances. In such a situation, the infusion should be stopped immediately.
In some cases, in severe cases, to support the kidneys in removing excess medicine, the doctor may need to put the patient on dialysis for a period of time.
To prevent such situations, the doctor regularly monitors the patient's condition and checks blood parameters.
In case of doubts about the use of the medicine, consult a doctor.
4. Possible side effects
Like all medicines, OLIMEL N12E can cause side effects, although not everybody gets them.
If you experience any changes in your condition during or after treatment, you should inform your doctor or nurse immediately.
Tests performed by the doctor during treatment should minimize the risk of side effects.
If unusual signs or symptoms of an allergic reaction occur, such as excessive sweating, fever, chills, headache, skin rash, or breathing difficulties, the infusion should be stopped immediately.
The following side effects have been reported during the use of OLIMEL N12E:
Frequency - Common: may affect up to 1 in 10 people
- rapid heartbeat (tachycardia);
- decreased appetite;
- increased fat levels in the blood (hypertriglyceridemia);
- abdominal pain;
- diarrhea;
- nausea;
- increased blood pressure (hypertension).
Frequency - Unknown: frequency cannot be estimated from the available data
- hypersensitivity reactions, including sweating, fever, chills, headache, skin rash (erythematous, papular, pustular, maculopapular, generalized rash), itching, flushing, breathing difficulties;
- infusion site reactions, which may lead to pain, irritation, swelling/edema, redness/warmth, skin necrosis, or blisters/bullae, inflammation, thickening, or tension of the skin at the infusion site;
- vomiting.
The following side effects have been reported with the use of similar parenteral nutrition products:
Frequency - Very rare: may affect up to 1 in 10,000 people
- fat overload syndrome, associated with sudden deterioration of the patient's condition. The symptoms of fat overload syndrome usually resolve after discontinuation of the fat emulsion:
- fever;
- decreased red blood cell count, which may cause pale skin and be the cause of weakness or shortness of breath (anemia);
- low white blood cell count, which may increase the risk of infection (leukopenia);
- low platelet count, which may increase the risk of bruising and/or bleeding (thrombocytopenia);
- coagulation disorders, which affect the ability of blood to clot;
- high blood fat levels (hyperlipidemia);
- fatty liver (hepatomegaly);
- impaired liver function;
- central nervous system symptoms (e.g., coma).
Frequency - Unknown: frequency cannot be estimated from the available data
- allergic reactions;
- abnormal liver function test results;
- difficulty in removing bile (cholestasis);
- enlargement of the liver (hepatomegaly);
- parenteral nutrition-associated liver disease (see "Warnings and precautions" in section 2);
- jaundice;
- low platelet count (thrombocytopenia);
- high blood nitrogen levels (azotemia);
- increased liver enzyme activity;
- formation of small particles that can block blood vessels in the lungs (pulmonary embolism), leading to respiratory failure.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, you should inform your doctor or nurse. Side effects can be reported directly to the Department of Drug Monitoring, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, PL 02-222 Warszawa, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store OLIMEL N12E, infusion emulsion
Store the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the container and outer packaging (MM/YYYY). The expiry date refers to the last day of the month.
Do not freeze.
Store in the protective packaging.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What OLIMEL N12E, infusion emulsion contains
The active substances in each bag of the ready-to-use emulsion are: 14.2% (corresponding to 14.2 g/100 ml) L-amino acid solution (alanine, arginine, glycine, histidine, isoleucine, leucine, lysine (in the form of lysine acetate), methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine, aspartic acid, glutamic acid) with electrolytes (sodium, potassium, magnesium, phosphates, acetates, chlorides), 17.5% (corresponding to 17.5 g/100 ml) fat emulsion (purified olive oil and purified soybean oil) and 27.5% (corresponding to 27.5 g/100 ml) glucose solution (in the form of glucose monohydrate) with calcium.
The other ingredients are:
| Fat emulsion chamber | Amino acid solution chamber | Glucose solution chamber |
| Purified egg phospholipids, glycerol, sodium oleate, sodium hydroxide (for pH adjustment), water for injection | Acetic acid (for pH adjustment), water for injection | Hydrochloric acid (for pH adjustment), water for injection |
What OLIMEL N12E, infusion emulsion looks like and contents of the pack
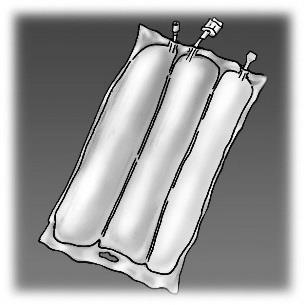
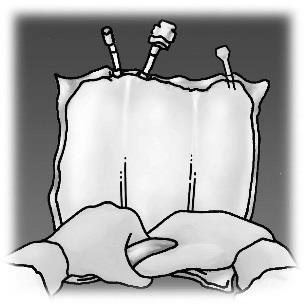
OLIMEL N12E is an infusion emulsion supplied in a triple-chamber bag. The first chamber contains the fat emulsion, the second chamber contains the amino acid solution with electrolytes, and the third chamber contains the glucose solution with calcium. The chambers are separated from each other by non-permeable welds. Before administration, the contents of the individual chambers should be mixed by rolling the bag in the direction of the welds, starting from the top of the bag, until the welds open.
Appearance before mixing:
- The amino acid and glucose solutions are clear, colorless, or slightly yellow.
- The fat emulsion is homogeneous with a milky appearance.
Appearance after mixing: homogeneous emulsion with a milky appearance.
The triple-chamber bag is a multi-layer plastic bag. The inner (contact) layer of the bag is compatible with the ingredients and permitted additives.
To prevent contact with oxygen in the air, the bag is packaged in a protective packaging that protects against oxygen, with an oxygen absorber sachet.
Pack sizes
650 ml bag: 1 cardboard box with 10 bags
1000 ml bag: 1 cardboard box with 6 bags
1500 ml bag: 1 cardboard box with 4 bags; 1 cardboard box with 5 bags
2000 ml bag: 1 cardboard box with 4 bags; 1 cardboard box with 5 bags
1 bag of 650 ml, 1000 ml, 1500 ml, and 2000 ml.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Baxter Polska Sp. z o.o.
ul. Kruczkowskiego 8
00-380 Warszawa
Manufacturer
BAXTER S.A.
Boulevard René Branquart 80
7860 Lessines
Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Estonia, France, Greece, Hungary, Italy, Latvia, Lithuania, Luxembourg, Netherlands, Romania, Slovenia, Slovakia, Spain: OLIMEL N12E
Austria: ZentroOLIMEL 7,6 % mit Elektrolyten
Germany: Olimel 7,6 % E
Denmark, Iceland, Sweden, Norway, Finland, Poland, Portugal: Olimel N12E
Ireland, Malta, United Kingdom: TRIOMEL 12 g/l nitrogen 950 kcal/l with electrolytes
Date of last revision of the leaflet: April 2025
Additional Ingredients
The bag capacity is sufficient to allow the addition of vitamins, electrolytes, and trace elements.
All additives (including vitamins) should be introduced into the ready-to-use emulsion (after breaking the welds and mixing the contents of the 3 chambers).
Vitamins can also be added to the glucose-containing chamber before preparing the ready-to-use emulsion (before breaking the welds and before mixing the contents of the three chambers).
Additional ingredients must be introduced by qualified personnel under aseptic conditions.
The OLIMEL N12E product can be supplemented with electrolytes, inorganic/organic phosphates, and commercially available multivitamin products (such as Cernevit) and products containing several trace elements (such as Nutryelt). The maximum total concentration of additional ingredients listed in the table below has been indicated based on stability data and should not be considered as dosage recommendations. Supplementation should be guided by the patient's clinical needs and should not exceed nutritional guidelines. When achieving the maximum total concentration, the amount of electrolytes already present in the bag should be taken into account.
Compatibility with products from different sources may vary, and healthcare professionals should perform appropriate checks when mixing the OLIMEL N12E product with other parenteral nutrition solutions.
| Possible additional ingredients per 1000 ml of OLIMEL N12E product (for children and adolescents) | |||
| Content | Maximum addition | Maximum content | |
| Sodium | 35 mmol | 115 mmol | 150 mmol |
| Potassium | 30 mmol | 120 mmol | 150 mmol |
| Magnesium | 4.0 mmol | 1.6 mmol | 5.6 mmol |
| Calcium | 3.5 mmol | 1.5 mmol | 5.0 mmol |
| Inorganic phosphates | 0 mmol | 10 mmol Pi or 10 mmol Pob | 10 mmol Pi + 15 mmol Po or 25 mmol Poa,b |
| Organic phosphates | 15 mmola | ||
| Other additional ingredients (trace elements, vitamins, selenium, and zinc)c | |||
| Trace elements - Nutryelt Pediatricd | 1 vial per bag (10 ml concentrate solution) | ||
| Vitamins e | 1 vial (lyophilisate) | ||
| Selenium | 60 µg per bag | ||
| Zinc | 3 mg per bag | ||
| Possible additional ingredients per 1000 ml of OLIMEL N12E product (for adults) | |||
| Content | Maximum addition | Maximum content | |
| Sodium | 35 mmol | 115 mmol | 150 mmol |
| Potassium | 30 mmol | 120 mmol | 150 mmol |
| Magnesium | 4.0 mmol | 1.6 mmol | 5.6 mmol |
| Calcium | 3.5 mmol | 1.5 mmol | 5.0 mmol |
| Inorganic phosphates | 0 mmol | 10 mmol Pi or 10 mmol Pob | 10 mmol Pi + 15 mmol Po or 25 mmol Poa,b |
| Organic phosphates | 15 mmola | ||
| Other additional ingredients (trace elements, vitamins, selenium, and zinc)c | |||
| Trace elements - Nutryelt d | 2 vials per bag (10 ml concentrate solution) | ||
| Vitamins - Cernevit | 1 vial (5 ml lyophilisate) | ||
| Selenium | 500 µg per bag | ||
| Zinc | 20 mg per bag | ||
When introducing additional ingredients, the following should be done:
- aseptic conditions should be controlled,
- the injection site on the bag should be prepared,
- the injection site should be punctured and the additional ingredients should be injected using a syringe needle or a device for preparing the medicinal product,
- the contents of the bag should be mixed with the additional ingredients.
Shelf-life after mixing
Chemical and physical stability has been demonstrated during use for 7 days at a temperature between 2°C and 8°C and then for 48 hours of storage at a temperature not exceeding 30°C.
From a microbiological point of view, the product should be used immediately. If the product is not used immediately, during use, the time and storage conditions until use are the responsibility of the user, and storage should not exceed 24 hours at a temperature between 2°C and 8°C, unless reconstitution took place in controlled and validated aseptic conditions.
Shelf-life after addition of additional ingredients
After the addition of additional ingredients, chemical and physical stability has been demonstrated during use for 7 days at a temperature between 2°C and 8°C and then for 48 hours of storage at a temperature not exceeding 30°C.
From a microbiological point of view, the product should be used immediately. If the product is not used immediately, during use, the time and storage conditions until use are the responsibility of the user, and storage should not exceed 24 hours at a temperature between 2°C and 8°C, unless the addition of additional ingredients took place in controlled and validated aseptic conditions.
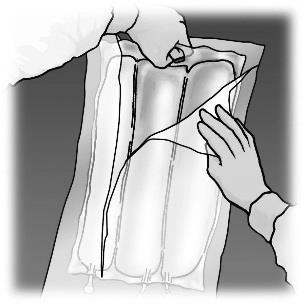
Preparation for infusion
Aseptic conditions should be controlled.
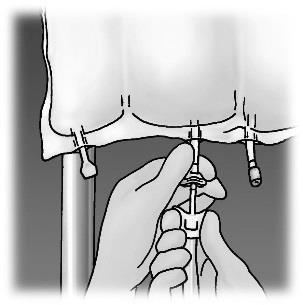
The bag should be hung.
The plastic protector should be removed from the port for administering the medicinal product.
The needle of the infusion set should be firmly inserted into the port for administering the medicinal product.
Figure 1. Stages of preparation of OLIMEL product for administration

Administration
For single use only.
The product should be administered only after breaking the welds separating the 3 chambers and mixing their contents.
It should be ensured that the ready-to-use emulsion for infusion does not separate into phases.
After opening the bag, the contents must be used immediately. The opened bag should not be stored for the next infusion. Partially used bags should not be reconnected.
To avoid the possibility of air embolism caused by the presence of gas in the first bag, bags should not be connected in series.
Any unused product or waste and the entire infusion set should be destroyed.
Extravasation
The site of catheter insertion should be regularly monitored for signs of extravasation.
In the event of extravasation, administration of the product should be stopped immediately, leaving the inserted catheter or cannula in place for immediate implementation of therapeutic measures. If possible, before removing the inserted catheter/cannula, aspiration of the liquid through the catheter/cannula should be performed to reduce the amount of liquid in the tissues.
Depending on the type of extravasated product (including products mixed with the OLIMEL N12E product, if applicable) and the degree/extent of potential injury, appropriate specific measures should be taken. Options for therapeutic measures may include non-pharmacological treatment, pharmacological treatment, and (or) surgical intervention. In the case of large extravasation, a plastic surgeon should be consulted within 72 hours.
The site of extravasation should be monitored at least every four hours during the first 24 hours and then once a day.
Infusion should not be resumed in the same central vein.
Baxter and Olimel are trademarks of Baxter International Inc.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBaxter S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Olimel N12eDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not required
Alternatives to Olimel N12e in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Olimel N12e in Hiszpania
Alternative to Olimel N12e in Ukraina
Online doctors for Olimel N12e
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Olimel N12e – subject to medical assessment and local rules.










