
How to use Olfen Path
Patient Information Leaflet: User Information
Warning! Keep the leaflet. Information on the immediate packaging in a foreign language.
Olfen Patch (Olfen)
140 mg, medicinal patch
Diclofenacum natricum
Olfen Patch and Olfen are different trade names for the same medicine.
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient information leaflet or as directed by your doctor or pharmacist.
- Keep this leaflet, you may need to read it again
- Ask your pharmacist if you need advice or further information
- If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4. If you do not feel better or feel worse after 3 days, contact your doctor.
Table of Contents of the Leaflet:
- 1. What is Olfen Patch and what is it used for
- 2. Important information before using Olfen Patch
- 3. How to use Olfen Patch
- 4. Possible side effects
- 5. How to store Olfen Patch
- 6. Contents of the pack and other information
1. What is Olfen Patch and what is it used for
Olfen Patch is a pain-relieving medicine. It belongs to a group of non-steroidal anti-inflammatory drugs (NSAIDs).
Olfen Patch is used locally for short-term therapy to relieve pain associated with sudden strains, sprains, or bruises in the hands and feet resulting from blunt injuries.
2. Important information before using Olfen Patch
When not to use Olfen Patch
- if you are allergic to diclofenac, propylene glycol, butylhydroxytoluene, or any of the other ingredients of this medicine (listed in section 6),
- if you are allergic to any other non-steroidal anti-inflammatory drug (NSAID, e.g., acetylsalicylic acid, ibuprofen),
- if you have had asthma attacks, skin rash, or irritation of the nose when taking acetylsalicylic acid or another NSAID,
- if you have an active stomach or duodenal ulcer,
- on wounds or open skin damage (e.g., abrasions, cuts, burns), in case of skin infections or eczema,
- during the last three months of pregnancy.
Warnings and precautions
Before starting to use Olfen Patch, discuss with your doctor or pharmacist:
- if you have or have had asthma or allergies, you may experience bronchospasm, which is characterized by difficulty breathing,
- if a rash appears; then you should immediately remove the patch and discontinue treatment,
- if you have kidney, heart, or liver problems or have a tendency to gastrointestinal ulcers, enteritis, or bleeding.
Side effects can be reduced by using the smallest possible doses for the shortest duration.
Important warnings:
- If symptoms do not improve after 3 days, contact your doctor.
- Do not apply the medicinal patch to the eyes or mucous membranes. Do not allow contact with the eyes and mucous membranes.
The patch should only be applied to undamaged skin. It should not be worn during bathing.
Avoid exposing the treated area, after removing the medicinal patch, to direct sunlight and sunlamps for about one day after removing the Olfen Patch, to reduce the risk of photosensitivity.
Do not use diclofenac-containing medicines and NSAIDs at the same time, either locally or systemically.
Olfen Patch and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as any medicines you plan to use. If Olfen Patch is used correctly, then only a very small amount of diclofenac penetrates the body, so interactions that are possible when taking the medicine orally should not occur.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
In the first six months of pregnancy, Olfen Patch should only be used after consulting a doctor.
During the last three months of pregnancy, do not use Olfen Patch due to the increased risk of complications for the mother and child (see "When not to use Olfen Patch").
Fertility
Olfen Patch should not be used in women who plan to become pregnant or have difficulty becoming pregnant, or are undergoing tests to determine the cause of infertility.
Breastfeeding
Before using any medicine, ask your doctor or pharmacist for advice.
Only small amounts of diclofenac pass into breast milk. In the case of short-term use of Olfen Patch, it is not necessary to interrupt breastfeeding, as no adverse effects have been observed in the infant.
However, Olfen Patch should not be applied near the breasts.
Driving and using machines
Olfen Patch has no or negligible influence on the ability to drive and use machines.
Olfen Patch contains propylene glycol and butylhydroxytoluene (E 321)
The medicine contains 1400 mg of propylene glycol in each patch. Propylene glycol may cause skin irritation.
Butylhydroxytoluene may cause local skin reactions (e.g., contact dermatitis) or eye and mucous membrane irritation.
3. How to use Olfen Patch
This medicine should always be used exactly as advised by your doctor. In case of doubt, consult your doctor or pharmacist.
Posology:
Apply the medicinal patch to the painful area twice a day, in the morning and evening. Do not use more than two medicinal patches per day, even if injuries occur in several places. Use only on one painful area at a time.
Olfen Patch should only be applied to undamaged, non-diseased skin.
Elderly patients
Elderly patients should use Olfen Patch with special caution, as they are more likely to experience side effects.
Patients with kidney, heart, or liver problems
In patients with kidney, heart, or liver problems, Olfen Patch should be used with special caution.
Children and adolescents under 16 years of age
There is a lack of sufficient data on the efficacy and safety of use in children and adolescents under 16 years of age.
Method of administration:
For cutaneous use only. Do not swallow!
- 1. Open the packaging containing the medicinal patch by cutting along the marked line.
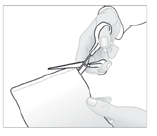
- 2. Remove the patch and close the packaging tightly, carefully pressing the edges.
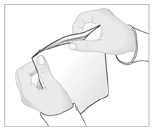
- 3. Remove the protective layer from the adhesive surface of the patch.
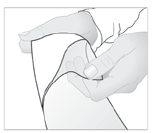
- 4. Apply the patch to the painful area.
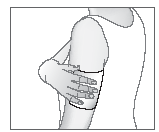
If necessary, a knitted bandage can be used to secure the patch in the desired position.
Do not use the medicinal patch under an airtight dressing (occlusive).
Do not divide the patch into parts.
Used patches should be folded in half, with the adhesive side inward.
Duration of treatment
Due to limited data, only short-term therapy is recommended.
Do not use Olfen Patch for more than 3 days without consulting a doctor.
The therapeutic benefits of using the patch for more than 7 days have not been confirmed.
In the case of adolescents aged 16 and adults, if it is necessary to use the product for more than 7 days to relieve pain or in case of worsening symptoms, parents or patients should consult a doctor.
Overdose of Olfen Patch
In case of severe side effects due to accidental overdose (e.g., by children) or incorrect use of Olfen Patch, contact a doctor. Based on the assessment of the degree of poisoning, the doctor will decide what remedial actions to take.
Missed dose of Olfen Patch
Do not use a double dose to make up for a missed dose.
If you have any further doubts about using the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Olfen Patch can cause side effects, although not everybody gets them.
Stop using the patch and consult a doctor immediately if you notice any of the following symptoms:
sudden itchy rash (hives), swelling of the hands, feet, ankles, face, lips, mouth, or throat, difficulty breathing, sudden drop in blood pressure, or feeling of weakness.
The following side effects may occur:
Frequently(may occur in 1 to 10 patients)
Reactions at the application site, rash, eruption, redness, skin inflammation (including allergic and contact dermatitis), skin swelling, itching, burning sensation.
Less frequently(may occur in 1 in 100 patients)
Generalized skin rash, allergic reactions (including hives), skin and mucous membrane swelling, anaphylactic reactions, photosensitivity.
Rarely(may occur in 1 to 10 in 1000 patients)
Blistering skin, dry skin.
Very rarely(may occur in 1 in 10,000 patients)
Asthma attack, severe eruption, pustular rash, rash with ulcers.
Frequency not known(frequency cannot be estimated from the available data)
Bleeding at the application site.
The absorption of diclofenac after topical application is very low, resulting in very low plasma concentrations of diclofenac compared to oral administration. The likelihood of systemic side effects (such as gastrointestinal disorders, liver or kidney problems, bronchospasm) is therefore negligible compared to oral diclofenac. However, if diclofenac is used for too long on large areas of skin, systemic side effects may occur.
If any of the side effects get worse or if you notice any side effects not listed in this leaflet, tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 (22) 49 21 301
fax: +48 (22) 49 21 309
website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Olfen Patch
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the month.
Store below 25°C. Store in the original packaging to protect from light and drying. Store the bag tightly closed to protect from drying.
Shelf life after opening the bag: 4 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Olfen Patch contains
The active substance is: diclofenac sodium. Each medicinal patch contains 140 mg of diclofenac sodium.
The other ingredients are: macrogol lauryl ether, diisopropyl adipate, glycerol, propylene glycol, liquid crystallizing sorbitol (E 420), sodium polyacrylate, sodium carmellose, butyl methacrylate copolymer, anhydrous colloidal silica, light natural kaolin, sodium metabisulfite (E 221), disodium edetate, butylhydroxytoluene (E 321), alum, tartaric acid, menthol, purified water, polyester backing layer, protective polypropylene film.
What Olfen Patch looks like and contents of the pack
Olfen Patch is a medicinal patch with dimensions of 10 cm x 14 cm with a uniform layer of adhesive substance applied to the dressing, white to light brown in color; protected by a protective layer.
Olfen Patch is available in packs containing 5 medicinal patches in 1 bag or 10 medicinal patches in 2 bags of 5 patches with the possibility of reclosing.
For more detailed information, contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Portugal, the country of export:
Mepha – Investigação, Desenvolvimento e Fabricação Farmacêutica, Lda.
Lagoas Park, Edifício 5-A, Piso 2, 2740-245 Porto Salvo
Portugal
Manufacturer:
Merckle GmbH
Ludwig-Merckle-Straße 3
89143 Blaubeuren
Germany
Teva Operations Poland Sp. z o.o.
ul. Mogilska 80
31-546 Kraków
Poland
Sofarimex – Indústria Química e Farmacêutica, S.A.
Avenida das Indústrias, Alto de Colaride – Agualva
2735-213 Cacém
Portugal
GlaxoSmithKline Santé Grand Public
23 rue Francois Jacob
92500 Rueil Malmaison
France
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Portuguese marketing authorization number, country of export: 5128723
Parallel import authorization number: 75/23
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
France:
VoltarenPlast 1%
Germany:
Olfen Patch
Poland:
Olfen Patch
Portugal:
Olfen
Date of leaflet approval: 27.04.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Teva B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Olfen PathDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Laboratorios Basi - Industria Farmaceutica, S.A.Prescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Sandoz GmbHPrescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacPrescription not required
Alternatives to Olfen Path in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Olfen Path in Hiszpania
Alternative to Olfen Path in Ukraina
Online doctors for Olfen Path
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Olfen Path – subject to medical assessment and local rules.







