
OLFEN 140 MG MEDICATED ADHESIVE PATCHES

How to use OLFEN 140 MG MEDICATED ADHESIVE PATCHES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Olfen 140 mg medicated adhesive patches
diclofenac sodium
Read the entire package leaflet carefully before starting to use the medicine, as it contains important information for you.
Follow the administration instructions of the medicine contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 3 days.
Contents of the package leaflet:
- What Olfen is and what it is used for
- What you need to know before using Olfen
- How to use Olfen
- Possible side effects
- Storage of Olfen
- Package contents and additional information
1. What Olfen is and what it is used for
Olfen is a medicine that reduces pain. It belongs to the group of non-steroidal anti-inflammatory drugs (NSAIDs).
For adults and adolescents from 16 years of age:
This medicine is used for short-term treatment of symptomatic local treatment of acute pain associated with sprains, strains, or bruises on arms and legs as a result of injuries, such as sports injuries.
2. What you need to know before using Olfen
Do not use Olfen
- if you are allergic to diclofenac, propylene glycol, butylhydroxytoluene, or any of the other components of this medicine (listed in section 6),
- if you are allergic to any other non-steroidal anti-inflammatory drug (NSAID, e.g., acetylsalicylic acid, ibuprofen)
- if you have developed asthma, skin rash, or inflammation and irritation inside the nose after taking acetylsalicylic acid or any other NSAID,
- if you have an active peptic ulcer,
- if you have open wounds on the skin (e.g., abrasions, cuts, burns), skin infections, or eczema,
- during the last three months of pregnancy.
- in children and adolescents under 16 years of age
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine
- if you have or have previously had bronchial asthma or allergies, you may experience bronchial muscle spasms (bronchospasm), which manifest with difficulty breathing,
- if you notice a skin rash, immediately remove the medicated patch and discontinue treatment,
- if you have kidney, heart, or liver disorders or if you have previously had gastrointestinal ulcers, intestinal inflammation, or a tendency to bleed.
Side effects can be reduced by using the minimum effective dose for the shortest possible time.
Important precautions:
- If symptoms persist for more than 3 days or worsen, you should consult your doctor,
Do not use the medicated patch on the eyes or mucous membranes or allow it to come into contact with them
After removing the medicated patch, avoid exposing the treated area directly to sunlight or other sources of ultraviolet rays (e.g., solarium) for at least one day to reduce the risk of sensitivity to light.
Do not use simultaneously, either topically or systemically, any medicine containing diclofenac or other NSAIDs
Elderly patients
Elderly patients should use this medicine with caution, as they are more likely to experience side effects.
Children and adolescents
This medicine should not be used in children and adolescents under 16 years of age due to lack of experience.
Using Olfen with other medicines
Tell your doctor or pharmacist if you are using/taking, have recently used, or may need to use/take any other medicine.
As long as the medicine is used correctly, the absorption of diclofenac by the body is very small, so the interactions described when diclofenac is administered orally are unlikely to occur.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
During the first six months of pregnancy, you can use Olfen medicated patches only after consulting your doctor.
During the last trimester of pregnancy, you should not use Olfen, as there is an increased risk of complications for the mother and child (see "Do not use Olfen").
Breastfeeding
Consult your doctor or pharmacist before using any medicine.
Very small amounts of diclofenac are excreted in breast milk. As no adverse effects are known in children, it is generally not necessary to interrupt breastfeeding during the use of Olfen. However, the patch should not be applied directly to the breast area.
Driving and using machines:
This medicine has a negligible influence on your ability to drive and use machines.
Olfen contains propylene glycol and butylhydroxytoluene,
This medicine contains 1,400 mg of propylene glycol in each patch. Propylene glycol may cause skin irritation.
Butylhydroxytoluene may cause local skin reactions (such as contact dermatitis) or eye and mucous membrane irritation.
3. How to use Olfen
Follow the administration instructions of the medicine contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Apply one medicated patch to the painful area twice a day, in the morning and at night. The maximum daily dose is 2 medicated patches, even if there are multiple areas to be treated. Treat only one painful area at a time.
Use in children and adolescents
There is not enough data on the efficacy and safety in children and adolescents under 16 years of age.
Method of administration
For cutaneous use only. Do not ingest!
- Cut the pouch containing the medicated patch along the mark.
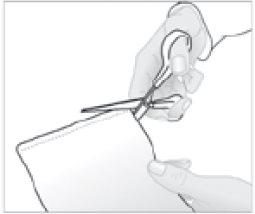
- Remove the medicated patch and carefully close the pouch by pressing the closure

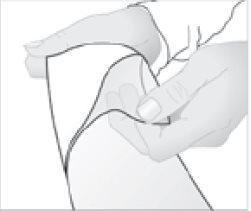
- Remove the protective film from the surface of the medicated patch.
- Place the medicated patch on the painful area.
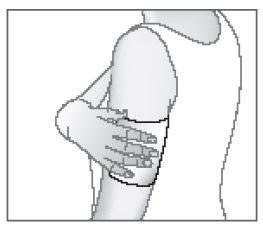
If necessary, the medicated patch can be adhered using an elastic bandage in a mesh form.
Do not use the medicated patch with an occlusive dressing.
Do not divide the medicated patch.
The used medicated patch should be folded in half, with the adhesive side facing inward.
Duration of use
Do not use Olfen for more than 3 days without consulting your doctor. The use of this medicine for a longer period should be consulted with the doctor and should not exceed 7 days.
If you use more Olfen than you should
If you have used more Olfen than you should, consult your doctor or pharmacist immediately or the Toxicology Information Service (Tel: 91 562 04 20).
Tell your doctor if you experience serious side effects after incorrect use of the medicine or after accidental overdose (e.g., in children). Your doctor will advise you on the necessary measures, depending on the severity of the poisoning.
If you forget to use Olfen
Do not use a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Consult your doctor immediately and discontinue the use of the medicated patch if you notice any of the following:
Sudden skin rash with itching (urticaria), swelling of the hands, feet, ankles, face, lips, mouth, or throat; difficulty breathing, low blood pressure, or weakness.
You may experience the following side effects:
Common (may affect up to 1 in 10 people)
Local skin reactions, such as redness of the skin, burning sensation, itching, redness of the inflamed skin, skin rashes, sometimes with pustules or papules.
Uncommon (may affect up to 1 in 100 people)
Hypersensitivity reactions or local allergic reactions (contact dermatitis).
Frequency not known (cannot be estimated from the available data)
Dry skin
In patients who have used topical active substances belonging to the same group of medicines as diclofenac, isolated cases of generalized skin rash, hypersensitivity reactions, such as skin and mucous membrane swelling, and anaphylactic reactions with acute circulatory disorders and sensitivity to light have been reported.
The absorption of diclofenac into the body through the skin is very low compared to the concentration of the active substance in the blood after oral ingestion of diclofenac. The probability of side effects in the body (such as kidney or gastrointestinal disorders or breathing difficulties) is very low.
If you think any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this package leaflet, tell your doctor or pharmacist.
Reporting of side effects:
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Olfen
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the package after the abbreviation EXP. The expiration date is the last day of the month indicated.
Do not store above 25°C.
Keep in the original packaging to protect from light and drying.
Store the pouch in a hermetically sealed condition to protect from drying.
It can be stored for 4 months after the first opening of the pouch.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package contents and additional information
Composition of Olfen
The active ingredient is: diclofenac sodium. Each medicated patch contains 140 mg of diclofenac sodium.
The other ingredients are: glycerol, propylene glycol (E1520), diisopropyl adipate, crystallizable sorbitol liquid (E-420), sodium carmellose, sodium polyacrylate, butyl methacrylate copolymer, disodium edetate, sodium sulfite (E-221), butylhydroxytoluene (E-321), aluminum potassium sulfate, anhydrous colloidal silica, light kaolin (natural), lauryl ether macrogol (9 EO units), levomenthol, tartaric acid, purified water, non-woven polyester backing, removable protective film of polypropylene.
Appearance of the product and package contents:
Olfen are patches of 10 x 14 cm with a paste of uniform white to light brown color as a uniform base on a non-woven backing and with a removable protective film.
Olfen is available in packages with 2, 5, 10, or 14 medicated patches in resealable pouches containing 2 or 5 medicated patches.
Not all package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Teva Pharma, S.L.U.
C/ Anabel Segura 11, Edificio
Albatros B 1ª planta 28108
Alcobendas, Madrid (Spain)
Manufacturer
Merckle GmbH
Ludwig-Merckle-Strabe 3
89143 Blaubeuren, (Germany)
This medicine is authorized in the EEA Member States under the following names:
Austria: Dolostrip 140 mg wirkstoffhaltiges Pflaster
Belgium: Kinespir Patch 140 mg pleister
Czech Republic: Olfen 140 mg lécivé náplasti
Denmark: Diclofenac ratiopharm
Germany: Diclofenac-ratiopharm Schmerzpflaster
Hungary: Algoplast-ratiopharm 140 mg gyógyszeres tapasz
Italy: Diclofenac Pharmentis 140mg cerotti medicati
Slovakia: Diclobene 140 mg
Spain: Olfen 140 mg apósitos adhesivos medicamentosos
United Kingdom: ALGOPAIN-Eze 140 mg medicated plaster
Date of last revision of this package leaflet:October 2019
Other sources of information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
You can access detailed and updated information about this medicine by scanning the QR code included in the packaging with your smartphone. You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/70307/P_70307.html
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OLFEN 140 MG MEDICATED ADHESIVE PATCHESDosage form: GEL, 10 mg/gActive substance: diclofenacManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: GEL, 23.2 mg/gActive substance: diclofenacManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: TOPICAL SOLUTION, 39.2 mg/mlActive substance: diclofenacManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for OLFEN 140 MG MEDICATED ADHESIVE PATCHES
Discuss questions about OLFEN 140 MG MEDICATED ADHESIVE PATCHES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






