
Ofloxamed

Ask a doctor about a prescription for Ofloxamed

How to use Ofloxamed
Package Leaflet: Information for the User
Ofloxamed, 3 mg/ml, Eye Drops, Solution
Ofloxacin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Ofloxamed and what is it used for
- 2. Important information before using Ofloxamed
- 3. How to use Ofloxamed
- 4. Possible side effects
- 5. How to store Ofloxamed
- 6. Contents of the pack and other information
1. What is Ofloxamed and what is it used for
Ofloxamed is an eye drop solution used to treat infections of the anterior segment of the eye, caused by organisms susceptible to ofloxacin.
Ofloxacin belongs to a group of medicines called quinolone antibacterials.
2. Important information before using Ofloxamed
When not to use Ofloxamed:
- if you are allergic to ofloxacin, other quinolones or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Ofloxamed, talk to your doctor or pharmacist:
- if you are planning to be exposed to excessive sunlight or UV radiation (e.g. sunlamps, solarium, etc.) while using Ofloxamed. You should avoid both sources of light due to the risk of photosensitivity;
- if you are allergic to other quinolone antibacterials;
- if you wear soft contact lenses. Do not wear contact lenses during eye infection (see "Ofloxamed contains 0.025 mg of benzalkonium chloride per milliliter").
Caution should be exercised when using the medicine in patients with damaged or ulcerated eye surface.
As with other antibiotics, long-term use of Ofloxamed may lead to the growth of non-susceptible pathogenic microorganisms. If such an infection occurs during treatment, the doctor will take appropriate measures.
Tendonitis and tendon rupture have occurred in patients taking fluoroquinolones orally or intravenously, especially in older patients and those treated with corticosteroids at the same time. Treatment with Ofloxamed should be discontinued if pain or inflammation of the tendon (tendinitis) occurs.
Children and adolescents
Safety and efficacy in infants under one year of age have not been established.
Before using the medicine in children, consult a doctor.
Ofloxamed and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about medicines you plan to take.
Because systemic exposure to ofloxacin is very low after using Ofloxamed, interactions with other medicines are not expected.
If other eye drops and/or ointments are used at the same time, a minimum interval of 15 minutes should be maintained between administration of the individual medicines. Eye ointments should always be used last.
Pregnancy and breastfeeding
Inform your doctor if you are pregnant or breastfeeding. Your doctor will advise you whether you can use this medicine.
Driving and using machines
Using Ofloxamed may cause blurred vision. These symptoms may persist for a few minutes. During this time, do not operate machines, work without strong support, or drive vehicles.
Ofloxamed contains 0.025 mg of benzalkonium chloride per milliliter
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Remove contact lenses before instillation and wait at least 15 minutes before reinserting.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or disorders affecting the cornea (the transparent layer at the front of the eye). If you experience any abnormal sensations in the eye, stinging, or pain in the eye after using the medicine, consult a doctor.
3. How to use Ofloxamed
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
For all patient groups, the recommended dose is:
1 dropof Ofloxamed
- every 2-4 hoursinto the conjunctival sac of the infected eye (s) for the first two days,
- then 4 times a day.
If the infection worsens or does not improve after a few days, consult a doctor.
Do not use this medicine for more than 14 days.
Note: If other eye drops and/or ointments are used at the same time, a minimum interval of 15 minutes should be maintained between administration of the individual medicines. Eye ointments should always be used last.
Instructions for use
Do not use the contents of the bottle if the tamper-evident seal on the bottle neck is broken before the first use. To open the bottle, unscrew the cap by turning it until the seal is broken.
The medicine should be instilled as follows:
- Wash your hands.
- Make sure the dropper tip does not touch the eye, eyelid, or surrounding area, or any other objects, to prevent contamination.
- Prepare a mirror and Ofloxamed.
- Open the bottle and hold it with the dropper facing down.
- Tilt your head back and pull the lower eyelid down, creating a pocket between the eyelid and the eye.
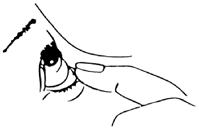
- Place the tip of the dropper very close to the eye. If necessary, you can use a mirror.
- Look up and instill one drop into the conjunctival sac by gently squeezing the bottle.
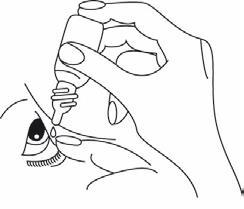
- Close your eye and press the corner of your eye near your nose for one minute. This will prevent the medicine from entering other parts of your body through the tear duct.
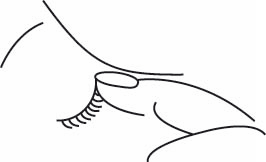
If you have been told to use the medicine in both eyes, repeat the above procedure for the second eye.
Immediately after use, close the bottle tightly with the cap to prevent contamination.
Wipe off any excess liquid from your cheek with a clean cloth.
Proper administration of eye drops is very important. If you are unsure, consult your doctor or pharmacist.
Using more Ofloxamed than you should
No cases of overdose have been reported.
If systemic side effects occur after incorrect use of the medicine or accidental overdose, systemic treatment should be initiated. In such cases, consult a doctor.
Forgetting to use Ofloxamed
Do not use a double dose to make up for a forgotten dose. Use the forgotten dose as soon as you remember, then continue using the medicine as instructed above or as advised by your doctor.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Ofloxamed can cause side effects, although not everybody gets them.
If you experience an allergic reaction (including eye allergic reaction), stop using the eye drops and consult a doctor immediately. This side effect is very rare (may affect up to 1 in 10,000 patients).
During treatment with Ofloxamed, life-threatening skin rashes (Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported, initially appearing as red spots or oval patches on the torso, often with blisters in the center. The frequency of this side effect is unknown (cannot be estimated from available data).
Photosensitivity reactions may also occur, manifesting as conjunctival redness and/or a burning sensation in the eye in response to light. However, these symptoms are usually short-lived. The frequency of this side effect is unknown (cannot be estimated from available data).
The following side effects may also occur:
- Frequently (may affect up to 1 in 10 patients):
- eye irritation,
- eye discomfort.
Rarely (may affect up to 1 in 1,000 patients):
- corneal deposits (especially in patients who have had corneal diseases before).
Frequency not known (cannot be estimated from available data):
- dizziness
- blurred vision
- photosensitivity
- eye swelling, face or around the eyes (including eyelid swelling)
- foreign body sensation in the eye
- tearing
- dry eye
- eye pain
- itching of the eyes or eyelids
- redness of the eyes
- nausea
- conjunctivitis
- keratitis.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
e-mail: [email protected]
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ofloxamed
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging and label after "EXP". The expiry date refers to the last day of that month.
Do not use after 4 weeks of opening.
Store the bottle in the outer packaging to protect from light.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Ofloxamed contains
- The active substance is ofloxacin. 1 ml of solution contains 3.0 mg of ofloxacin. One dose (1 drop) contains 0.1 mg of ofloxacin.
- The other ingredients are: sodium chloride, benzalkonium chloride, hydrochloric acid, and sodium hydroxide solution (to adjust pH), water for injections.
What Ofloxamed looks like and contents of the pack
Ofloxamed is a clear, yellowish solution.
Each bottle contains 5 ml of eye drop solution.
The product is available in the following pack sizes:
1 x 5 ml
3 x 5 ml
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
SUN-FARM Sp. z o.o.
ul. Dolna 21
05-092 Łomianki
Manufacturer
mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
Date of last revision of the leaflet:11.2019
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- Importermibe GmbH Arzneimittel
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OfloxamedDosage form: Ointment, 3 mg/gActive substance: ofloxacinManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription requiredDosage form: Drops, 3 mg/ml (0.3%)Active substance: ofloxacinManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription required
Alternatives to Ofloxamed in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ofloxamed in Ukraine
Alternative to Ofloxamed in Spain
Online doctors for Ofloxamed
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ofloxamed – subject to medical assessment and local rules.














