CETRAFLUX 3 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS


How to use CETRAFLUX 3 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
CETRAFLUX 3 mg/ml eye drops, solution in single-dose container
{Active substance(s)}
Read the entire package leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet.
Contents of the package leaflet
- What is Cetraflux and what is it used for
- What you need to know before you start using Cetraflux
- How to use Cetraflux
- Possible side effects
- Storing Cetraflux
- Contents of the pack and further information
1. What is Cetraflux and what is it used for
Cetraflux is an eye drop.
Ciprofloxacin belongs to a group of antibiotics called quinolones and a subgroup called fluoroquinolones.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or common cold. It is essential to follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste. |
Cetraflux is used to treat infections caused by bacteria in the eye(s): purulent bacterial conjunctivitis, keratitis (bacterial inflammation of the cornea), corneal ulcers, and corneal abscesses.
2. What you need to know before you start using Cetraflux
Do not use Cetraflux
If you are allergic to ciprofloxacin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- Use this medicine only in your eye(s). Do not inject it, do not swallow it.
- If you experience severe allergic reactions, rash, hives, itching, or difficulty breathing, discontinue treatment immediately and consult your doctor, as this may occur after the first dose.
- If symptoms worsen or recur quickly, consult your doctor. With the use of this medicine, you may become more susceptible to other infections.
- If, during or shortly after using eye drops, you experience pain, swelling, or inflammation of the tendons, discontinue treatment and consult your doctor.
- If you wear contact lenses:
- Wearing contact lenses (hard or soft) is not recommended during the treatment of an eye infection.
- If you are taking any other medicine, see the section on Using Cetraflux with other medicines.
Using Cetraflux with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
It is preferable to avoid this medicine during pregnancy unless the therapeutic benefit outweighs the potential risk to the fetus.
Ciprofloxacin passes into breast milk. If you are breastfeeding a baby, consult your doctor before using Cetraflux.
Driving and using machines
If you notice that your vision is altered in any way after using Cetraflux, do not drive or operate machinery.
Important information if you wear contact lenses
It is not recommended to wear contact lenses during the treatment of an eye infection, as your infection could worsen. Do not use the eye drops if you are wearing contact lenses.
Wait at least 15 minutes after using the eye drops before putting your contact lenses back in.
If you have any further questions about using Cetraflux, ask your doctor or pharmacist.
3. How to use Cetraflux
Follow the instructions for administering this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The recommended dose will be different depending on whether you are being treated for corneal abscesses or other bacterial eye infections.
Adults, newborns, children, and adolescents
Corneal abscesses
The eye drops should be administered at the following intervals, even during the night.
- On the first day, instill two drops into the affected eye every 15 minutes for the first six hours, and then two drops every 30 minutes for the rest of the day.
- On the second day, instill two drops into the affected eye every hour.
- From the third day to the 14th, instill two drops into the affected eye every 4 hours.
If it is necessary to continue treatment with this medicine after the 14th day, your doctor will indicate this.
Other eye infections:
- For the first two days, instill one or two drops into the affected eye(s) every 2 hours during the day while you are awake.
- From the third day onwards, instill one or two drops every 4 hours during the day.
Your doctor will indicate the duration of the treatment.
It is essential to continue the treatment with Cetraflux until the end, as indicated by your doctor.
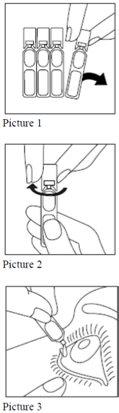
Recommendations for use
Always use Cetraflux exactly as instructed by your doctor.
Consult your doctor or pharmacist if you have any doubts.
|
|
If a drop falls outside the eye, try again.
If you use more Cetraflux eye drops than you should
You can rinse your eyes with lukewarm water. Do not apply more drops until it is time for your next dose.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
If you forget to use Cetraflux eye drops
Do not use a double dose to make up for it. Apply a single dose as soon as you remember, and then return to your regular schedule. If it is almost time for your next dose, do not apply the missed dose; continue with your next scheduled dose.
4. Possible side effects
Like all medicines, Cetraflux can cause side effects, although not everybody gets them.
You may experience some or all of the following side effects in your eye:
Common (may affect up to 1 in 10 people): deposits in the cornea, eye discomfort, ocular hyperemia (eye redness).
Uncommon (may affect up to 1 in 100 people): keratopathy (non-inflammatory disease of the cornea), corneal infiltrates, corneal staining, photophobia (sensitivity to light), decreased visual acuity (decreased vision), eyelid edema (swollen eyelid), blurred vision, eye pain, dry eye, eye swelling, ocular pruritus (itching in the eye), foreign body sensation in the eyes (feeling of having something in the eye), increased tearing (watery eyes), ocular discharge, crusts on the eyelid margin, eyelid exfoliation, conjunctival edema, eyelid erythema (redness of the inner eyelid).
Rare (may affect up to 1 in 1,000 people): ocular toxicity, punctate keratitis (destruction of the outer areas of the cornea), keratitis (inflammation of the cornea), conjunctivitis, corneal disorder, corneal epithelial defect, diplopia (double vision), ocular hypoesthesia (decreased sensitivity of the eye), asthenopia (tired vision), ocular irritation, ocular inflammation, conjunctival hyperemia. If you notice white particles in your eyes, continue using Cetraflux but inform your doctor immediately.
You may experience effects in other areas of your body, such as:
Infections and infestations:
Rare (may affect up to 1 in 1,000 people): hordeolum (stye), rhinitis (inflammation of the mucous membrane inside the nose).
Immune system disorders:
Rare (may affect up to 1 in 1,000 people): allergic reactions such as skin rash, itching, swelling, difficulty breathing.
Nervous system disorders:
Common (may affect up to 1 in 10 people): bad taste in the mouth.
Uncommon (may affect up to 1 in 100 people): headache.
Rare (may affect up to 1 in 1,000 people): dizziness.
Ear and labyrinth disorders:
Rare (may affect up to 1 in 1,000 people): ear pain.
Respiratory, thoracic, and mediastinal disorders:
Rare (may affect up to 1 in 1,000 people): hypersecretion of the paranasal sinuses.
Gastrointestinal disorders:
Uncommon (may affect up to 1 in 100 people): nausea.
Rare (may affect up to 1 in 1,000 people): diarrhea, abdominal pain.
Skin and subcutaneous tissue disorders:
Rare (may affect up to 1 in 1,000 people): dermatitis (skin inflammation).
General disorders and administration site conditions:
Rare (may affect up to 1 in 1,000 people): drug intolerance.
Investigations:
Rare (may affect up to 1 in 1,000 people): abnormal laboratory test results.
If you think any of the side effects you are experiencing is serious or if you notice any side effects not listed in this package leaflet, tell your doctor or pharmacist.
If you experience an allergic reaction, do not use Cetraflux and consult your doctor.
5. Storing Cetraflux
Keep this medicine out of the sight and reach of children.
Do not store above 30°C. Keep the ampoule in the outer packaging to protect it from light.
Do not use this medicine after the expiry date (EXP) stated on the carton. The expiry date is the last day of the month stated.
Shelf life after opening the single-dose container: the contents of the ampoule should be used immediately once opened. Any remaining amount should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Cetraflux
- The active substance is ciprofloxacin. Each ml of solution contains 3 mg of ciprofloxacin (as hydrochloride).
- The other ingredients are mannitol, disodium edetate, glacial acetic acid, sodium acetate trihydrate, hydrochloric acid, and purified water.
Appearance of the product and contents of the pack
Cetraflux is a sterile, preservative-free aqueous solution, clear, colorless, or slightly yellowish, presented in packs of 40 ampoules. Each ampoule provides 0.25 ml. The ampoules should be kept inside the bag for protection.
Marketing authorization holder and manufacturer
Laboratorios Salvat, S.A.
Gall 30 - 36
08950 Esplugues de Llobregat (Barcelona)
This medicine is authorized in the Member States of the European Economic Area under the following names:
Cetraflux 3mg/ml Colírio, solução | Portugal Laboratorios Salvat, S.A. Gall 30 - 36 08950 Esplugues de Llobregat (Barcelona) |
Cetraflux 3mg/ml collirio, soluzione | Italy Laboratorios Salvat, S.A. Gall 30 - 36 08950 Esplugues de Llobregat (Barcelona) |
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
Date of the last revision of this package leaflet:
- Country of registration
- Average pharmacy price4.64 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CETRAFLUX 3 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: OPHTHALMIC OINTMENT, 3.5 gramsActive substance: ciprofloxacinManufacturer: Ntc S.R.L.Prescription requiredDosage form: EYE DROP, 3.5 mg ciprofloxacin hydrochlorideActive substance: ciprofloxacinManufacturer: Ntc S.R.L.Prescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for CETRAFLUX 3 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about CETRAFLUX 3 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions