
Noqturina
Ask a doctor about a prescription for Noqturina

How to use Noqturina
Leaflet accompanying the packaging: patient information
Noqturina, 25 micrograms, oral lyophilisate
Noqturina, 50 micrograms, oral lyophilisate
Desmopressin
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor. See section 4.
Table of contents of the leaflet:
- 1. What is Noqturina and what is it used for
- 2. Important information before taking Noqturina
- 3. How to take Noqturina
- 4. Possible side effects
- 5. How to store Noqturina
- 6. Contents of the pack and other information
1. What is Noqturina and what is it used for
Noqturina contains desmopressin, an antidiuretic agent that reduces urine production.
Noqturina is used to treat nocturia (the need to urinate frequently at night) caused by nocturnal polyuria (excessive urine production at night) in adults.
2. Important information before taking Noqturina
When not to take Noqturina
- if you are allergic to desmopressin or any of the other ingredients of this medicine (listed in section 6);
- if you have polydipsia (excessive thirst and increased fluid intake) or psychogenic polydipsia (excessive thirst and increased fluid intake due to psychological factors);
- if you have heart failure (the heart is not able to pump enough blood);
- if you have any diseases that require the use of diuretics;
- if you have moderate or severe kidney impairment;
- if you have or have had hyponatremia (low sodium levels in the blood);
- if you have SIADH (inappropriate secretion of antidiuretic hormone).
Warnings and precautions
Before starting treatment with Noqturina, discuss it with your doctor.
Your doctor will exercise caution in the following cases:
- if you have severe urinary bladder disorders and difficulty urinating;
- if you are 65 years of age or older, as your doctor will need to monitor your sodium levels (see section 3, "How to take Noqturina" below);
- if you have low sodium levels in your blood;
- if you have a condition that affects water and/or electrolyte balance;
- if you have a condition that may worsen due to water and/or electrolyte imbalance;
- if you develop an acute illness (such as a generalized infection, a disease with fever, gastroenteritis) during treatment, as your doctor may need to interrupt treatment with Noqturina;
- if you have cystic fibrosis, coronary heart disease, hypertension, chronic kidney disease, or pre-eclampsia.
Fluid intake should be limited to a minimum from 1 hour before taking Noqturina to 8 hours after taking Noqturina. Treatment without concurrent fluid restriction may lead to excessive water retention and/or electrolyte imbalance, which may cause symptoms such as headache, nausea, vomiting, weight gain, or, in severe cases, seizures.
Noqturina and other medicines
Tell your doctor about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
In particular, inform your doctor if you are taking:tricyclic antidepressants, used to treat depression (such as clomipramine, imipramine, desipramine);
selective serotonin reuptake inhibitors, used to treat depression or anxiety disorders (such as citalopram, paroxetine, sertraline);
chlorpromazine, an antipsychotic used to treat schizophrenia;
diuretics (such as thiazides or other types of diuretics);
carbamazepine, used to treat bipolar affective disorder and epilepsy;
antidiabetic medicines used to treat type 2 diabetes (sulfonylureas), especially chlorpropamide;
non-steroidal anti-inflammatory drugs used to treat pain and inflammation (such as acetylsalicylic acid and ibuprofen);
oxytocin, used during childbirth;
lithium, used to treat bipolar affective disorder;
loperamide, used to treat diarrhea.
Noqturina with food and drink
Noqturina should not be taken with food, as it may reduce the therapeutic effect.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before taking this medicine.
Your doctor will decide whether you can take this medicine while pregnant or breastfeeding.
Driving and using machines
Noqturina has no or negligible influence on the ability to drive and use machines.
3. How to take Noqturina
Always take this medicine exactly as your doctor has told you.
If you are unsure, ask your doctor.
The recommended dose is:
- for women: 25 micrograms per day, 1 hour before bedtime, taken sublingually, without drinking water;
- for men: 50 micrograms per day, 1 hour before bedtime, taken sublingually, without drinking water.
Noqturina should be placed under the tongue, where it dissolves without the need for water.
Instructions for taking the medicine
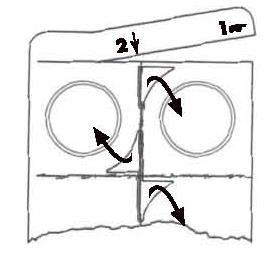
- 1.Completely remove the end tab of the blister by tearing it off along the perforation, starting from the corner with the printed hand symbol.
- 2.Tear off one unit of the blister along the perforation.
- 3.Remove the foil from the torn unit of the blister, starting from the corner with the printed arrow and pulling the foil in the direction indicated by the arrow. Do not press the lyophilisate through the foil.
- 4.Gently remove the lyophilisate from the unit of the blister. Place the lyophilisate under the tongue and let it dissolve. Do not chew or swallow the lyophilisate.
- 5.If the lyophilisate breaks into more than two pieces during removal from the unit of the blister, do not take the broken pieces. Take a lyophilisate from another unit of the blister.
Fluid intake should be limited to a minimum from 1 hour before taking Noqturina to 8 hours after taking Noqturina. If you experience any of the following symptoms, stop treatment and contact your doctor: headache, nausea, vomiting, weight gain, or, in severe cases, seizures (see "Warnings and precautions" above). Your doctor may recommend resuming treatment. When resuming treatment, it is necessary to strictly limit fluid intake. Additionally, your doctor will closely monitor your sodium levels.
Use in elderly patients (65 years and older)
In patients 65 years of age or older, your doctor will need to monitor your sodium levels before starting treatment, during the first week of treatment (4-8 days after starting treatment), and again after about one month of treatment.
Use in patients with renal impairment
If you have moderate or severe kidney impairment, do not take Noqturina.
Consult your doctor.
Use in patients with hepatic impairment
If you have liver function disorders, consult your doctor before taking Noqturina.
Use in children and adolescents
This medicine is intended for use only in adults.
Overdose of Noqturina
It is important not to take more than the prescribed dose in any 24-hour period.
Particular attention should be paid to signs of water intoxication (hyponatremia), such as weight gain, headache, nausea, and, in severe cases, seizures.
If you take more than the recommended dose of Noqturina, consult your doctor.
Missed dose of Noqturina
Do not take a double dose to make up for a missed dose. From the next day, continue taking the medicine according to the established schedule.
Stopping treatment with Noqturina
Treatment can only be stopped or discontinued in consultation with your doctor.
If you have any further questions about taking this medicine, ask your doctor.
4. Possible side effects
Like all medicines, Noqturina can cause side effects, although not everybody gets them.
Drinking excessive amounts of fluid may lead to water accumulation, which in severe cases can dilute the sodium in the body. This can be a serious condition and lead to seizures.
Stop taking the medicine and immediately inform your doctor or go to the emergency department if you experience one or more of the following symptoms:
- extremely severe or prolonged headache,
- confusion,
- unexplained weight gain,
- nausea or vomiting.
Side effects include:
Very common:may affect more than 1 in 10 people:
- dry mouth.
Common:may affect up to 1 in 10 people:
- nausea, malaise, muscle weakness, and confusion due to decreased sodium levels in the blood (hyponatremia),
- headache,
- dizziness,
- nausea,
- diarrhea.
Uncommon:may affect up to 1 in 100 people:
- constipation,
- stomach problems,
- weakness,
- swelling of the tissues in the lower limbs (peripheral edema).
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309;
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Noqturina
Keep the medicine out of the sight and reach of children.
There are no special storage instructions for the medicine.
Store in the original packaging to protect from moisture and light.
Use immediately after opening the unit blister with the lyophilisate.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Noqturina contains
- The active substance is desmopressin in the form of desmopressin acetate. One oral lyophilisate contains 25 micrograms of desmopressin. One oral lyophilisate contains 50 micrograms of desmopressin.
- The other ingredients are: gelatin, mannitol (E 421), and anhydrous citric acid.
What Noqturina looks like and contents of the pack
Noqturina 25 micrograms:
A white, round lyophilisate tablet with a diameter of about 12 mm, marked with 25 on one side.
Noqturina 50 micrograms:
A white, round lyophilisate tablet with a diameter of about 12 mm, marked with 50 on one side.
Blisters in a cardboard box. One blister, divided into single doses, contains 10 oral lyophilisates.
Pack sizes:
10x1, 30x1, 90x1, or 100x1 oral lyophilisates.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Ferring GmbH
Wittland 11
D-24109 Kiel
Germany
Manufacturer:
Ferring GmbH
Wittland 11
D-24109 Kiel
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Nocdurna 25/50 microgram – Lyophilisate for oral use
Belgium
Nocdurna 25/50 lyophilisate for oral use
Bulgaria
Нокдурна 25/50 микрограма перорален лиофилизат
Croatia
Nocdurna 25/50 microgram oral lyophilisate
Cyprus
Nocdurna
Denmark
Nocdurna
Estonia
Nokdirna
Finland
Nocdurna
France
Nocdurna 25/50 micrograms, lyophilisate oral
Greece
Nocdurna Δισκίο λυοφιλοποιημένο, από του στόματος 25mcgΠAB (Γνωστή δραστική)
Nocdurna Δισκίο λυοφιλοποιημένο, από του στόματος 50mcgΠAB (Γνωστή δραστική)
Netherlands
Nocdurna 25/50 microgram
Ireland
Noqturina 25/50 microgram oral lyophilisate
Iceland
Nocdurna
Liechtenstein
Nocdurna 25/50 mikrogramm – Lyophilisat zum Einnehmen
Lithuania
Nokdirna
Luxembourg
Nocdurna 25 mcg lyophilisate oral/50 microgram oral lyophilisate
Latvia
Nokdirna 25/50 mikrogrami liofilizāts iekšķīgai lietošanai
Malta
Noqturina 25/50 microgram oral lyophilisate
Germany
Nocdurna
Norway
Nocdurna
Poland
Noqturina
Portugal
Nocdurna
Czech Republic
Nocdurna
Romania
Nocdurna 25/50 micrograme liofilizat oral
Slovakia
Nocdurna
Slovenia
Nocdurna 25/50 mikrogramov peroralni liofilizat
Sweden
Nocdurna
Hungary
Nocdurna
United Kingdom
Noqdirna 25/50 microgram oral lyophilisate
Date of last revision of the leaflet: 01/2019
For more detailed information, please consult the representative of the marketing authorization holder.
Ferring Pharmaceuticals Poland Sp. z o.o.
Szamocka 8
01-748 Warsaw
Tel.: + 48 22 246 06 80, Fax: + 48 22 246 06 81
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterFerring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NoqturinaDosage form: Solution, 20 IU/mlActive substance: vasopressin (argipressin)Prescription not requiredDosage form: Concentrate, 40 IU/2 mlActive substance: vasopressin (argipressin)Prescription not requiredDosage form: Lyophilizate, 50 mcgActive substance: vasopressin (argipressin)Prescription required
Alternatives to Noqturina in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Noqturina in Ukraine
Alternative to Noqturina in Spain
Online doctors for Noqturina
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Noqturina – subject to medical assessment and local rules.














