
Netaxen
Ask a doctor about a prescription for Netaxen

How to use Netaxen
Package Leaflet: Information for the Patient
Netaxen 3 mg/mL +1 mg/mL,
eye drops, solution
nethylmycin + dexamethasone
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Contents of the Package Leaflet
- 1. What is Netaxen and what is it used for
- 2. Important information before using Netaxen
- 3. How to use Netaxen
- 4. Possible side effects
- 5. How to store Netaxen
- 6. Contents of the pack and other information
1. What is Netaxen and what is it used for
Netaxen contains two active substances: nethylmycin and dexamethasone.
- Nethylmycin is an antibiotic that kills bacteria.
- Dexamethasone is a corticosteroid that reduces inflammation.
Netaxen is used in adults to reduce inflammation and kill bacteria in the eye, where there is swelling and irritation and where a bacterial infection is likely to occur.
If there is no improvement after finishing the treatment or if you feel worse, you should contact your doctor.
2. Important information before using Netaxen
Netaxen can be used in adults, including the elderly.
The medicine is not recommended for use in children under 18 years of age.
When not to use Netaxen:
- if you are allergic to nethylmycin, dexamethasone, aminoglycoside antibiotics (such as tobramycin, kanamycin, amikacin, gentamicin, etc.) or any of the other ingredients of this medicine (listed in section 6).
- if your doctor has told you that the pressure in your eye is too high.
- if you think you may have a viral or fungal infection in or around your eye.
- if you have or have had a viral infection of the eye caused by the herpes simplex virus.
- if your doctor has told you that you have an eye infection caused by tuberculosis.
Warnings and precautions
Before starting treatment with Netaxen, discuss it with your doctor or pharmacist.
You should talk to your doctor if you experience swelling and weight gain around the trunk and face, as these are usually the first symptoms of a condition called Cushing's syndrome. After stopping long-term or intensive treatment with Netaxen, there may be a suppression of adrenal function. Before stopping treatment with this medicine, you should discuss it with your doctor. These risks are particularly important in children and patients treated with ritonavir or cobicistat.
If you experience blurred vision or other vision disturbances, you should contact your doctor.
If you use Netaxen for a long time:
- the pressure in your eye may increase and cause damage to the nerves in your eye, and then vision problems. If you use Netaxen for more than 15 days, your doctor should regularly check the pressure in your eye;
- you may develop cataracts;
- wounds may heal more slowly;
- your body may not fight other types of eye infections, such as fungal or viral, as effectively as usual;
- eye infections with significant discharge after using corticosteroids may worsen or be more difficult to identify the type of bacteria causing the infection;
- the steroid in Netaxen may cause thinning of the surface of the eye, and even perforation;
- you may become allergic to the antibiotic in the eye drops.
Before using Netaxen, tell your doctor if:
- you have glaucoma or glaucoma occurs in your family;
- you have problems with the cornea;
- you are taking any other medicines that contain phosphates. Your doctor will regularly check your cornea.
- you wear contact lenses. People wearing contact lenses can use Netaxen provided they follow the instructions described in section 3.
Children and adolescents
Netaxen is not recommended for use in children and adolescents (from birth to 18 years of age).
For external use only.
Netaxen should only be used on the surface of the eye. This medicine should never be injected or swallowed.
Netaxen and other medicines
Netaxen may interact with other medicines. Tell your doctor or pharmacist if you are taking or have recently taken any other eye product or other medicines, including those obtained without a prescription. Netaxen can be used with other eye products provided you follow the instructions described in section 3.
Tell your doctor or pharmacist if you are taking:
- other antibiotics, especially polymyxin B, colistin, viomycin, streptomycin, vancomycin, or cephaloridine; Using Netaxen with other antibiotics may increase the risk of kidney problems, hearing problems, or affect the effectiveness of other antibiotics;
- cisplatin (a medicine used to treat cancer);
- diuretics (medicines that prevent water retention in the body) such as ethacrynic acid and furosemide;
- anticholinergic medicines (medicines that inhibit the secretory activity of glands) such as atropine;
- ritonavir or cobicistat, as this may increase the amount of dexamethasone in your blood;
- other medicines that contain phosphates. Your doctor will regularly check your cornea.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Use during pregnancy
Use of Netaxen is not recommended during pregnancy, unless your doctor considers it necessary.
Use during breastfeeding
Netaxen should not be used during breastfeeding.
Driving and using machines
After using Netaxen, temporary blurred vision may occur. Do not drive or operate machinery until your vision is clear.
Netaxen contains benzalkonium chloride and phosphates.
This medicine contains 0.05 mg of benzalkonium chloride in each mL.
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. You should remove your contact lenses before using Netaxen eye drops and wait at least 15 minutes before putting them back.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or disorders related to the cornea (the transparent layer at the front of the eye). If you experience any abnormal sensations in the eye, stinging, or pain in the eye after using this medicine, you should contact your doctor.
This medicine contains 0.18 mg of phosphates in each drop, which corresponds to 3.66 mg/mL. If you have serious damage to the transparent front layer of the eye (cornea), phosphates may, in very rare cases, cause clouding of the cornea due to calcium deposits during treatment.
3. How to use Netaxen
This medicine should always be used exactly as your doctor or pharmacist has told you.
If you are not sure, ask your doctor or pharmacist.
The recommended dose is one dropinto the affected eye four times a dayor as directed by your doctor. The duration of treatment is usually between 5 and 14 days.
Do not change the dose of the eye drops without consulting your doctor.
Use in children and adolescents
Netaxen is not recommended for use in children and adolescents (from birth to 18 years of age).
Contact lenses
If you wear contact lenses, you should remove them before using Netaxen eye drops. After using Netaxen, wait 15 minutes before putting your contact lenses back.
If you need to use Netaxen while wearing contact lenses, use Netaxen in single-dose containers without preservatives.
However, you should not wear contact lenses during treatment with steroid eye drops due to the increased risk of infection. You should also avoid wearing contact lenses during eye infections or inflammatory conditions.
Using Netaxen with other eye drops
Wait at least 10 minutes between using Netaxen and using other eye drops or ointments. Eye ointments should be used last.
Instructions for use
Check that the bottle is intact.
- 1. Wash your hands and sit comfortably.
- 2. Tighten the cap firmly to pierce the tip of the bottle. Unscrew the cap.
- 3. Tilt your head back.
- 4. Gently pull down the lower eyelid of the affected eye. 5 Turn the bottle upside down and place the dropper tip close to the eye, without touching the eye. Do not touch the eye or eyelid with the tip of the bottle.
- 6. Gently squeeze the bottle to release only one drop, then release the lower eyelid.
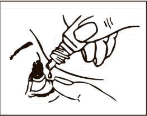
- 7. Close your eye and press the corner of your eye near your nose for 2 minutes.
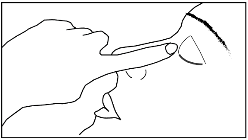
8 Repeat in the other eye as directed by your doctor.
9 Close the bottle with the cap.
Improperly used eye drops can become contaminated with bacteria, which can lead to eye infections. Using contaminated eye drops can cause serious eye damage and vision loss.
Using more Netaxen than recommended
If you accidentally put more drops in your eye than recommended, it is unlikely to cause problems.
Use the next dose at the usual time.
If you swallow the entire contents of Netaxen, you should contact your doctor immediately, as side effects may occur.
Missing a dose of Netaxen
Do not use a double dose to make up for a missed dose.
Use the next dose at the usual time.
Stopping treatment with Netaxen
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Netaxen can cause side effects, although not everybody gets them.
The frequency of the following side effects cannot be estimated from the available data.
Eye disorders
Increased pressure in the eye, cataract formation after long-term treatment, blurred vision, occurrence or worsening of viral eye infections caused by the herpes simplex virus or fungal infections, delayed wound healing.
In very rare cases (less than 1 in 10,000) in some patients with severe damage to the transparent front layer of the eye (cornea), cloudy spots appeared on the cornea due to calcium deposits during treatment.
Immune system disorders
Local allergic reactions: conjunctival redness, burning, itching.
Endocrine disorders
Excessive hair growth on the body (especially in women), muscle weakness and wasting, purple stretch marks on the skin, high blood pressure, irregular menstrual periods or absence of menstruation, changes in protein and calcium levels in the body, growth retardation in children and adolescents, and swelling and weight gain around the trunk and face (Cushing's syndrome) (see section 2, "Warnings and precautions").
In all the above cases, treatment should be discontinued.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, PL-02-222 Warsaw, Tel.: + 48 22 49 21 301, Fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Netaxen
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and bottle after EXP. The expiry date refers to the last day of that month.
Store in a temperature below 30°C.
Shelf life after first opening the bottle: 28 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Netaxen contains
- The active substances of Netaxen are nethylmycin and dexamethasone. Each mL of solution contains 4.55 mg of nethylmycin sulfate (equivalent to 3 mg of nethylmycin) and 1.32 mg of dexamethasone sodium phosphate (equivalent to 1 mg of dexamethasone).
- The other ingredients are: sodium citrate, sodium dihydrogen phosphate monohydrate, disodium phosphate dodecahydrate, benzalkonium chloride, purified water.
What Netaxen looks like and contents of the pack
Netaxen is a clear, colorless or slightly yellowish solution.
A LDPE bottle with a screw cap in a cardboard box. 1 bottle contains 5 mL of Netaxen eye drops, solution.
Marketing authorization holder and manufacturer
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci S. Antonio (CT)
Italy
[email protected]
This medicine is authorized in the Member States of the European Economic Area under the following names:
Bulgaria, Germany, Latvia: Netildex
Greece, Spain: NETDEX
Cyprus, Czech Republic, Lithuania, Slovakia: Netaxan
Portugal: Dexamethasone + Netilmicin SIFI
France: NETAXEN
Poland: Netaxen
Date of last revision of the leaflet:
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products - www.urpl.gov.pl.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSifi S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NetaxenDosage form: Drops, (3 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Rafarm S.A.Prescription requiredDosage form: Drops, (5 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription requiredDosage form: Ointment, (5 mg + 0.3 mg)/gActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription required
Alternatives to Netaxen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Netaxen in Ukraine
Alternative to Netaxen in Spain
Online doctors for Netaxen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Netaxen – subject to medical assessment and local rules.














