Minirin Melt

Ask a doctor about a prescription for Minirin Melt

How to use Minirin Melt
Leaflet accompanying the packaging: patient information
MINIRIN Melt, 60 micrograms, oral lyophilisate
MINIRIN Melt, 120 micrograms, oral lyophilisate
MINIRIN Melt, 240 micrograms, oral lyophilisate
Desmopressin
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor. See section 4.
Table of contents of the leaflet:
- 1. What is Minirin Melt and what is it used for
- 2. Important information before taking Minirin Melt
- 3. How to take Minirin Melt
- 4. Possible side effects
- 5. How to store Minirin Melt
- 6. Contents of the packaging and other information
1. What is Minirin Melt and what is it used for
Minirin Melt contains desmopressin, which acts similarly to the natural hormone of the pituitary gland, arginine vasopressin. Desmopressin is characterized by a significantly prolonged antidiuretic effect (reducing urine production) and a complete lack of vasoconstrictive effect at therapeutic doses. Minirin Melt is used to treat:
- central diabetes insipidus;
- primary nocturnal enuresis in children over 6 years of age with normal urine concentration ability;
- nocturia in adults associated with nocturnal polyuria (the volume of urine produced at night exceeds the bladder capacity).
2. Important information before taking Minirin Melt
When not to take Minirin Melt
- if the patient is allergic to desmopressin or any of the other ingredients of this medicine (listed in section 6);
- if the patient has been diagnosed with psychogenic or habitual polydipsia (excessive thirst);
- if the patient has heart failure or other diseases that require the administration of diuretics;
- if the patient has moderate or severe renal impairment with a creatinine clearance below 50 ml/min;
- if the patient has hyponatremia (low sodium levels in the blood);
- if the patient has been diagnosed with the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Warnings and precautions
Before starting treatment with Minirin Melt, the patient should discuss it with their doctor. The doctor will exercise caution in the following cases:
- if the medicine is taken simultaneously with other medicines; see the chapter "Concomitant use of other medicines";
- if the patient is over 65 years old;
- if the patient has low sodium levels in the blood;
- if there is a risk of increased intracranial pressure;
- if the patient has a water and/or electrolyte imbalance.
Before starting treatment, severe urinary bladder dysfunction and obstruction should be ruled out. Special precautions should be taken in patients with renal impairment and cardiovascular disease. In the event of acute diseases with water and electrolyte disorders, such as generalized infection, feverish diseases, gastroenteritis, the administration of Minirin Melt should be discontinued and the doctor consulted.
Children
Minirin Melt is used to treat central diabetes insipidus and primary nocturnal enuresis in children over 6 years of age with normal urine concentration ability.
Minirin Melt and other medicines
The patient should inform their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. In particular, the patient should inform their doctor about taking: tricyclic antidepressants; selective serotonin reuptake inhibitors; chlorpromazine; carbamazepine; oral hypoglycemic agents from the sulfonylurea group, e.g. chlorpropamide; non-steroidal anti-inflammatory drugs; loperamide. The above-mentioned medicines may lead to excessive water retention in the body or a decrease in sodium levels in the blood.
Minirin Melt with food and drink
During treatment of primary nocturnal enuresis in children and nocturia in adults, fluid intake should be limited to a minimum from 1 hour before taking the medicine to 8 hours after taking it. Taking Minirin Melt without restricting fluid intake may lead to excessive water retention in the body or a decrease in sodium levels in the blood, which may, but does not have to, manifest as headaches, nausea, vomiting, weight gain, or in severe cases, convulsions. The above warning does not apply to patients taking Minirin Melt for the treatment of central diabetes insipidus. Taking the medicine with food may reduce the strength and duration of its effect.
Minirin Melt in patients with renal and/or hepatic impairment
Before taking the medicine, the patient should consult their doctor.
Minirin Melt in elderly patients
It is not recommended to start treatment for nocturia in patients over 65 years old.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before taking this medicine.
Driving and using machines
Minirin Melt has no influence or negligible influence on the ability to drive and use machines.
3. How to take Minirin Melt
This medicine should always be taken according to the doctor's recommendations. In case of doubts, the patient should consult their doctor. Minirin Melt should be placed under the tongue, where the medicine dissolves without the need to drink water. Administration of Minirin Melt in the treatment of central diabetes insipidus The doctor determines the dosage individually for each patient, but the total daily dose is usually within the range of 120 to 720 micrograms sublingually. Usually, treatment of children and adults starts with 60 micrograms of desmopressin sublingually 3 times a day. The next doses are determined by the doctor depending on the patient's response. In most patients, the maintenance dose is 60 to 120 micrograms of desmopressin sublingually 3 times a day. Administration of Minirin Melt in the treatment of primary nocturnal enuresis in children Usually, treatment starts with a dose of 120 micrograms. The medicine is administered sublingually once a day before bedtime. If this dose is insufficient, the doctor may prescribe an increase in the dose to 240 micrograms. Fluid intake should be limited to a minimum from 1 hour before taking the medicine to 8 hours after taking it. After three months of treatment, the doctor should prescribe a break in the administration of the medicine for at least one week and assess whether further treatment is necessary. Administration of Minirin Melt in the treatment of nocturia in adults Usually, treatment starts with a dose of 60 micrograms. The medicine is administered sublingually once a day before bedtime. If this dose is not sufficiently effective after one week of treatment, the doctor may prescribe an increase in the dose to 120 micrograms and then to 240 micrograms, with weekly intervals when increasing the dose. Fluid intake should be limited to a minimum from 1 hour before taking the medicine to 8 hours after taking it.
Taking a higher dose of Minirin Melt than recommended
Taking a higher dose of the medicine than recommended increases the risk of excessive water retention in the body or a decrease in sodium levels in the blood, which may, but does not have to, manifest as headaches, nausea, vomiting, weight gain, or in severe cases, convulsions. In case of taking a higher dose of the medicine than recommended, the patient should immediately consult their doctor.
Missing a dose of Minirin Melt
The patient should not take a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, Minirin Melt can cause side effects, although not everybody gets them. In adults:
- Very common side effectsaffect more than 1 in 10 people taking the medicine:
Very common side effectsinclude:
- headache.
Common side effectsaffect 1 to 10 people in 100:
- hyponatremia (low sodium levels in the blood);
- dizziness;
- hypertension;
- nausea;
- abdominal pain;
- diarrhea;
- constipation;
- vomiting;
- symptoms related to the urinary bladder and urethra;
- edema;
- fatigue.
Uncommon side effectsaffect 1 to 10 people in 1,000:
- insomnia;
- drowsiness;
- paresthesia (tingling, prickling, or numbness);
- impaired vision;
- balance disorders;
- palpitations;
- orthostatic hypotension (decrease in blood pressure when changing position from lying to standing);
- dyspnea;
- indigestion;
- flatulence;
- bloating;
- sweating;
- itching;
- rash;
- urticaria;
- muscle cramps;
- muscle pain;
- malaise;
- chest pain;
- flu-like symptoms;
- weight gain;
- increased liver enzyme levels;
- hypokalemia (low potassium levels in the blood).
Rare side effectsaffect 1 to 10 people in 10,000:
- confusion;
- allergic skin reactions.
Frequency not known(cannot be estimated from the available data):
- anaphylactic reactions;
- dehydration;
- hypernatremia (high sodium levels in the blood);
- seizures;
- weakness;
- coma.
In children and adolescents:
- Common side effectsaffect 1 to 10 people in 100:
Common side effectsinclude:
- headache.
Uncommon side effectsaffect 1 to 10 people in 1,000:
- emotional instability;
- aggression;
- abdominal pain;
- nausea;
- vomiting;
- diarrhea;
- symptoms related to the urinary bladder and urethra;
- peripheral edema;
- fatigue.
Rare side effectsaffect 1 to 10 people in 10,000:
- restlessness;
- nightmares;
- mood swings;
- drowsiness;
- hypertension;
- irritability.
Frequency not known(cannot be estimated from the available data)
- anaphylactic reactions;
- hyponatremia;
- abnormal behavior;
- emotional disorders;
- depression;
- hallucinations;
- insomnia;
- attention disorders;
- psychomotor hyperactivity;
- seizures;
- epistaxis;
- allergic skin reactions;
- rash;
- sweating;
- urticaria.
Reporting side effects
If any side effects occur, including those not listed in the leaflet, the patient should inform their doctor. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Aleje Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Minirin Melt
The medicine should be stored out of sight and reach of children. Store in the original packaging to protect from moisture and light. Do not use this medicine after the expiry date stated on the packaging after the words EXP. The expiry date refers to the last day of the month. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Minirin Melt contains
- The active substance of the medicine is desmopressin in a quantity of 60, 120, or 240 micrograms.
- The other ingredients are: gelatin, mannitol, anhydrous citric acid.
What Minirin Melt looks like and contents of the packaging
Minirin Melt 60 micrograms is a white tablet with a drop-shaped marking on one side. Minirin Melt 120 micrograms is a white tablet with a marking in the shape of two drops on one side. Minirin Melt 240 micrograms is a white tablet with a marking in the shape of three drops on one side. One packaging of Minirin Melt contains 30 or 100 oral lyophilisates.
Marketing authorization holder and manufacturer
Marketing authorization holder: Ferring-Léčiva, a.s., K Rybníku 475, 252-42 Jesenice u Prahy, Czech Republic. Manufacturer: Ferring GmbH, Wittland 11, D-24109 Kiel, Germany. Date of last revision of the leaflet:10/2020. For more detailed information, please contact the representative of the marketing authorization holder. Ferring Pharmaceuticals Poland Sp. z o.o., ul. Szamocka 8, 01-748 Warsaw, Tel.: +48 22 246 06 80, Fax: +48 22 246 06 81
Instructions for removing lyophilisates from the blister pack
Lyophilisates are fragile. Do not push them through the blister pack foil, as this may cause them to crumble. Lyophilisates should be removed from the blister pack after removing the aluminum foil, as shown in the diagrams below:
- 1.Tear off the end strip of the blister pack, starting from the corner with the printed symbol of a hand.
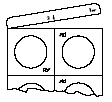
- 2.Tear off the unit of the blister pack along the perforation.
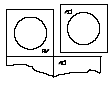
- 3.Pull back the foil, starting from the corner with the printed arrow, and gently remove the lyophilisate.
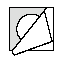
- 4.By repeating steps 2 and 3, access to the next lyophilisate is obtained.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFerring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Minirin MeltDosage form: Lyophilizate, 60 mcgActive substance: desmopressinManufacturer: Ferring GmbHPrescription requiredDosage form: Lyophilizate, 120 mcgActive substance: desmopressinManufacturer: Ferring GmbHPrescription requiredDosage form: Lyophilizate, 240 mcgActive substance: desmopressinManufacturer: Ferring GmbHPrescription required
Alternatives to Minirin Melt in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Minirin Melt in Spain
Alternative to Minirin Melt in Ukraine
Online doctors for Minirin Melt
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Minirin Melt – subject to medical assessment and local rules.