
DESMIRIN 360 micrograms/ml ORAL SOLUTION

How to use DESMIRIN 360 micrograms/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Desmirin 360 micrograms/ml oral solution
(Desmopressin)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Desmirin and what is it used for
- What you need to know before you take Desmirin
- How to take Desmirin
- Possible side effects
- Storing Desmirin
- Contents of the pack and other information
1. What is Desmirin and what is it used for
The active substance that makes Desmirin work (the active ingredient) is desmopressin. Desmopressin is very similar to a substance that is produced naturally in the body (the pituitary hormone vasopressin), which temporarily reduces the amount of urine produced by the body. This medicine is for oral use only.
Desmirin is used to treat:
- Central diabetes insipidus, a disease that causes excessive thirst and the continuous production of large amounts of diluted urine as a result of insufficient production of the vasopressin hormone.
Bedwetting (involuntary urination at night or primary nocturnal enuresis) in patients over 5 years of age with normal ability to concentrate urine.
2. What you need to know before you take Desmirin
Do not take Desmirin
- If you are allergic (hypersensitive) to desmopressin or any of the other ingredients of this medicine (listed in section 6),
- If you unusually drink large amounts of fluids (you have habitual or psychogenic polydipsia),
- If you take medicines that increase urine production (diuretics),
- If you have heart problems,
- If you have a predisposition or have low sodium levels in the blood (hyponatremia),
- If you have kidney problems,
- If you have uncontrolled high blood pressure,
- If you have the "Syndrome of Inadequate Secretion of the Antidiuretic Hormone" (SIADH).
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine.
During treatment with Desmirin, avoid drinking excessive amounts of fluids because it can cause water retention in the body and/or low sodium levels in the blood with or without side effects (see section 4 Possible side effects).
Special care should be taken to avoid water retention in the body and low sodium levels in the blood in the following cases:
- if you are an elderly person,
- if you have a medical condition that causes fluid and/or electrolyte imbalance in the body, such as an infection, fever, or stomach upset.
- if you have severe bladder problems or alteration of urine flow,
- if you have asthma, epilepsy, and migraine,
- if you have kidney problems and/or cardiovascular diseases.
- In chronic kidney disease, the antidiuretic effect of this medicine is less than usual.
Taking Desmirin with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, especially:
- tricyclic antidepressantsor SSRIs(used to treat depression),
- carbamazepine(used to treat epilepsy),
- chlorpromazine(used to treat psychosis or schizophrenia),
- medicines for pain and/or inflammationcalled non-steroidal anti-inflammatory drugs (NSAIDs), for example indomethacin, ibuprofen, acetylsalicylic acid,
- loperamide(used to treat diarrhea),
- diuretic agents.
These medicines increase the risk of fluid retention, which dilutes salt in the body.
- Dimethicone(used in the treatment of gas accumulation), due to decreased absorption of desmopressin.
Taking Desmirin with food and drinks
At low doses, Desmirin may be affected by food intake. If you notice that this medicine is less effective, you should take it without food before increasing the dose.
When using this medicine for bedwetting, reduce fluid intake to a minimum, from 1 hour before taking Desmirin to 8 hours after taking a dose.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
It is preferable to avoid the use of this medicine during pregnancy.
Desmopressin passes into breast milk. If you are treated with desmopressin, you should stop breastfeeding.
Driving and using machines
Desmirin does not affect the ability to drive or use machines.
Important information about some of the ingredients of Desmirin
This medicine contains methyl parahydroxybenzoate and propyl parahydroxybenzoate, which may cause allergic reactions (possibly delayed).
3. How to take Desmirin
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Usual dose
Treatment of central diabetes insipidus
Adults and children:Your doctor will adjust the dose individually. The initial doserecommended is 0.25 ml (90 micrograms) three times a day. Subsequently, the doctor will adjust the dose according to the response of each patient. The usual daily doseranges from 0.5 ml (180 micrograms) to 3 ml (1080 micrograms) of Desmirin. The maintenance doseusually ranges from 0.25 to 0.5 ml (90-180 micrograms) of Desmirin three times a day.
It is important to observe if symptoms of water retention in the body and/or low sodium levels in the blood appear (see section 4 Possible side effects). In this case, treatment will be interrupted and the dose will be adjusted again.
Bedwetting (involuntary urination at night or primary nocturnal enuresis) in patients over 5 years of age:
Adults and children:the usual initial dose is 0.5 ml (180 micrograms) of Desmirin one hour before bedtime. If this is not sufficiently active, the dose of Desmirin can be increased to 1 ml (360 micrograms). The need to continue treatment is usually checked every three months, alternating with a period without treatment for at least one week.
Elderly:if the doctor decides to treat you, sodium levels in the blood should be measured before and three days after starting treatment and if the dose is increased or at any time your doctor considers it appropriate.
It is important to control fluid intake. If symptoms of water retention in the body and/or low sodium levels in the blood appear (see section 4 Possible side effects), treatment will be interrupted. Once treatment is restarted, fluid intake will be strictly controlled.
Instructions for use:
- Open the bottle (when opening for the first time, the seal will break).
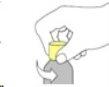
- Insert the oral syringe into the adapter and turn the bottle upside down to fill it with the dose to be administered
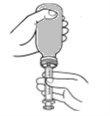
- Remove the oral syringe from the bottle and check that the correct amount is inside the syringe.
- Hold the syringe in the mouth and release the dose into the mouth.
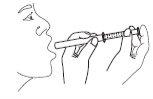
- Rinse with water after each use and close the bottle. Keep the bottle in the original packaging to protect it from light.
If you take more Desmirin than you should
In case of overdose or accidental ingestion, contact your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, indicating the medicine and the amount ingested.
An overdose can prolong the effect of desmopressin and increase the risk of fluid retention in the body and/or low sodium levels in the blood. Symptoms may include headache, nausea, vomiting, weight gain, and in severe cases, convulsions. It is recommended to interrupt treatment, restrict fluid intake, and provide symptomatic treatment if necessary.If you forget to take Desmirin
Do not take a double dose to make up for forgotten doses.
If you stop taking Desmirin
Do not stop taking Desmirin before completing the treatment, as it may not have the expected effect. You should only change or stop the treatment if your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking Desmirin and go to see a doctor or the nearest hospital if you experience any of the following:
- Frequent(may affect up to 1 in 10 people): symptoms due to fluid retention in the body, such as unusual or prolonged headache, feeling or being sick, unexplained weight gain, and in severe cases, convulsions, loss of consciousness.
- Very rare(may affect up to 1 in 10,000 people): allergic reactions such as rash, itching, fever, swelling of the lips, face, throat, or tongue causing difficulty swallowing or breathing.
Tell your doctor or pharmacistif you notice any of the following side effects:
- Very frequent(may affect more than 1 in 10 people): headache
- Frequent(may affect up to 1 in 10 people): abdominal pain, nausea.
- Very rare(may affect up to 1 in 10,000 people): emotional disorders in children, allergic reactions.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Desmirin
Keep this medicine out of the sight and reach of children.
Do not store above 30°C. Store in the original packaging.
After the first opening, the medicine should be stored below 25°C for up to 4 weeks.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help to protect the environment.
6. Contents of the pack and other information
Composition of Desmirin
The active substance is desmopressin. Each ml of oral solution contains 360 micrograms of desmopressin (as desmopressin acetate).
The other ingredients are:
- Methyl parahydroxybenzoate sodium (E-219),
- Propyl parahydroxybenzoate sodium (E-217),
- Hydrochloric acid (for pH adjustment) and
- Purified water.
Appearance of Desmirin and contents of the pack
Desmirin is a clear solution introduced in an amber glass bottle with a low-density polyethylene (LDPE) adapter, provided with a high-density polyethylene (HDPE) cap. The bottle contains 15 ml of solution. A 1.5 ml graduated syringe is supplied with each pack. The syringe is graduated from 0 to 1.5 ml, with divisions every 0.1 ml. The graduations corresponding to the doses of 0.25 ml, 0.5 ml, and 1.0 ml are specifically marked.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Laboratorio Reig Jofré, S.A.
Gran Capitán, 10
08970 Sant Joan Despí - Barcelona
Spain
Tel.: +34 934806719
Fax: +34 934806724
Manufacturer
Laboratorio Reig Jofré S.A.
Gran Capitán 10
08970 Sant Joan Despí - Barcelona
Spain
Tel.: +34 934806719
Fax: +34 934806724
This medicine is authorised in the Member States of the European Economic Area under the following names:
Spain: Desmirin 360 micrograms/ml oral solution
Date of last revision of this leaflet:November 2019
Other sources of information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DESMIRIN 360 micrograms/ml ORAL SOLUTIONDosage form: SUBLINGUAL TABLET, 120 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: SUBLINGUAL TABLET, 240 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: SUBLINGUAL TABLET, 60 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for DESMIRIN 360 micrograms/ml ORAL SOLUTION
Discuss questions about DESMIRIN 360 micrograms/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















