
Midanium

Ask a doctor about a prescription for Midanium

How to use Midanium
Leaflet accompanying the packaging: patient information
MIDANIUM, 1 mg/ml, solution for injection
MIDANIUM, 5 mg/ml, solution for injection
Midazolam
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so you can read it again if you need to.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Midanium and what is it used for
- 2. Important information before using Midanium
- 3. How to use Midanium
- 4. Possible side effects
- 5. How to store Midanium
- 6. Contents of the packaging and other information
1. What is Midanium and what is it used for
Midanium contains the active substance midazolam. Midazolam belongs to a group of medicines called benzodiazepines. It is characterized by a short-acting, strong sedative and hypnotic effect.
Midanium is used in adults:
- -for sedation (calming, relieving anxiety and restlessness) while maintaining consciousness before and during short diagnostic and therapeutic procedures;
- -in patient preparation (premedication) before various procedures, e.g., before surgery, endoscopy;
- -as a sedative component to induce general anesthesia, before using another anesthetic agent;
- -for sedation in intensive care units.
Midanium is used in children:
- -for sedation (calming, relieving anxiety and restlessness) while maintaining consciousness before and during short diagnostic and therapeutic procedures;
- -in patient preparation (premedication) before various procedures, e.g., before surgery, endoscopy;
- -for sedation in intensive care units.
2. Important information before using Midanium
When not to use Midanium:
- if the patient is allergic to midazolam (or other benzodiazepines) or any of the other ingredients of this medicine (listed in section 6);
- if the patient has severe respiratory failure or acute respiratory depression (very serious breathing difficulties).
Warnings and precautions
Before starting treatment with Midanium, discuss it with your doctor or nurse.
Consult your doctor in the case of patients:
- -over 60 years old, weakened;
- -with liver function disorders, chronic liver failure, or kidney failure;
- -with chronic respiratory failure;
- -in a severe condition, especially with heart diseases and breathing difficulties;
- -with myasthenia (a chronic disease characterized by muscle weakness). Patients with these diseases should inform their doctor.
After receiving Midanium, the patient should be discharged from the hospital or treatment room only with the doctor's consent. The patient should be accompanied after discharge. Do not use the medicine for a long time, as it may increase the risk of dependence and the development of tolerance to the medicine. The development of tolerance is characterized by the increasingly weaker effect of the medicine over time, which requires an increase in the dose.
Children
- -In infants under 6 months of age, Midanium should only be used in intensive care units.
- -During the use of Midanium in children, so-called paradoxical reactions (symptoms listed in section 4 "Possible side effects") may occur. If these symptoms occur, the use of the medicine should be stopped.
- -In children with heart diseases (especially with unstable circulation), special caution should be exercised.
Midanium and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Midanium and other medicines used at the same time may affect each other's effects.
This is especially true for the following medicines listed below:
- -antifungal medicines (ketoconazole, voriconazole, fluconazole, itraconazole, posaconazole);
- -macrolide antibiotics (erythromycin, clarithromycin);
- -HIV protease inhibitors (medicines used in HIV infection - saquinavir);
- -verapamil and diltiazem (calcium channel blockers, mainly used in cardiovascular diseases);
- -atorvastatin (a medicine that lowers blood cholesterol levels);
- -aprepitant (an antiemetic medicine);
- -rifampicin (an antibiotic);
- -phenytoin (an antiepileptic medicine);
- -efavirenz (a medicine used in HIV infection);
- -strong painkillers (called opioid painkillers, e.g., morphine, buprenorphine);
- -medicines used in mental disorders (e.g., haloperidol, carbamazepine);
- -sedatives, anxiolytics, and hypnotics;
- -medicines used in the treatment of depression (fluoxetine, fluvoxamine);
- -medicines used in the treatment of allergies (with a sedative effect);
- -hydralazine (a medicine for hypertension);
- -inhaled anesthetics used for general anesthesia;
- -herbal mixtures containing St. John's Wort extract ( Hypericum perforatum).
Concomitant use of Midanium and opioids (strong painkillers, substitution therapy drugs, and some cough medicines) increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can be life-threatening. For this reason, concomitant use of these medicines can only be considered when other treatment options are not possible.
If, however, the doctor has prescribed Midanium together with opioid medicines, the doctor should reduce the dose and recommend the shortest possible treatment time.
You should inform your doctor about all opioid medicines you are taking and strictly follow the doctor's instructions regarding dosing. It may be helpful to inform friends or relatives about the risk, so they are aware of the above symptoms. If such symptoms occur, you should contact your doctor.
Midanium and alcohol
Alcohol (ethanol) significantly enhances the sedative effect of midazolam. Consuming alcohol during treatment with Midanium is contraindicated.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine.
Midazolam may only be used during pregnancy if absolutely necessary.
Midazolam is not recommended during cesarean section.
Midazolam passes into human milk in small amounts. If the medicine needs to be used, the patient should stop breastfeeding and resume after 24 hours from the administration of midazolam.
Driving and using machines
After using the medicine, the following may occur: sedation, amnesia, concentration disorders, muscle weakness, which may adversely affect the performance of tasks that require increased attention.
Do not drive vehicles or operate machines until you have fully recovered. The doctor will decide when you can resume these activities.
Midanium contains sodium
The medicine contains 3.16 mg of sodium (the main component of table salt) per 1 ml. This corresponds to 0.16% of the maximum recommended daily sodium intake in the diet for adults.
The medicine may be diluted - see below "Method of preparation of Midanium for administration and method of administration". When calculating the total sodium content in the prepared dilution of the medicine, the sodium from the diluent should be taken into account. To obtain accurate information about the sodium content in the solution used for dilution, you should read the patient leaflet of the diluent used.
3. How to use Midanium
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor.
- -Midanium is administered by medical personnel.
- -The medicine is used intramuscularly, intravenously, and rectally.
- -The doctor will determine the dosage of the medicine and the duration of treatment individually for each patient.
Detailed information on dosing is contained in the section "Information intended only for healthcare professionals".
Using a higher dose of Midanium than recommended
The medicine is administered by medical personnel, so it is unlikely that the patient will receive more medicine than they should. In case of midazolam overdose, the following may occur: drowsiness, clumsiness, speech disorders or slurred speech, and nystagmus. In severe cases, loss of reflexes, apnea, hypotension, severe breathing difficulties, and in rare cases - coma.
If you think you have received a higher dose of the medicine than recommended, you should immediately consult your doctor, who will provide appropriate treatment.
If you miss a dose of Midanium
Do not take a double dose to make up for a missed dose.
Stopping the use of Midanium
In the case of long-term use of midazolam in intensive care units, after sudden cessation of the medicine, withdrawal syndrome may occur. The symptoms of the syndrome include: headaches, muscle pain, anxiety, tension, restlessness, confusion, irritability, recurring insomnia, mood changes, hallucinations, and seizures.
The doctor will choose the right dose and duration of treatment to minimize the risk of these symptoms.
If you have any further doubts about the use of this medicine, consult your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur during the use of this medicine. Their frequency is unknown and cannot be determined based on available data.
You should stop using Midanium and immediately consult your doctor if you experience any of the following side effects. They may be life-threatening and require immediate treatment:
- anaphylactic shock (a life-threatening allergic reaction). Symptoms may include sudden rash, itching, or hives and swelling of the face, lips, tongue, or other parts of the body. The patient may also experience shortness of breath, wheezing, or breathing difficulties, or pallor, weak and rapid pulse, or a feeling of loss of consciousness. Additionally, chest pain may occur, which may be a symptom of a severe allergic reaction called Kounis syndrome.
- shortness of breath and breathing difficulties (sometimes leading to respiratory arrest), apnea;
- laryngospasm causing choking;
- heart disorders, such as bradycardia, vasodilation, cardiac arrest.
Other side effects, the frequency of which is unknown (the frequency cannot be determined based on available data):
- confusion, euphoria, hallucinations;
- excitement, feelings of hostility and anger, aggression, outbursts of excitement, involuntary movements (including clonic-tonic seizures and muscle tremors), excessive activity, violent acts (so-called paradoxical reactions, especially in children and the elderly); physical dependence and withdrawal symptoms, abuse of the medicine, seizures (in preterm and newborn infants, also as withdrawal symptoms);
- sedation (excessive calming) lasting longer and postoperative, decreased level of consciousness, drowsiness, dizziness; clumsiness; anterograde amnesia (the patient does not remember events that occurred shortly after administration of the medicine) - the duration of these side effects depends on the dose used.
- hiccups;
- vasodilation;
- hypotension;
- dryness of the mucous membrane of the mouth, nausea, vomiting, constipation;
- skin rash, urticaria, itching;
- severe venous diseases (thrombophlebitis and venous thrombosis);
- pain and redness at the injection site;
- fatigue;
- increased risk of falls and fractures in elderly patients and patients taking sedatives.
Prolonged intravenous administration of midazolam, even in therapeutic doses, may lead to the development of physical dependence, and sudden cessation of the medicine may be accompanied by withdrawal syndrome symptoms, including seizures - see the "Stopping the use of Midanium" section.
In elderly patients and weakened patients, especially those with heart diseases and breathing difficulties, more severe side effects may occur, especially after too rapid injection or administration of large doses.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181 C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Midanium
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging and ampoule. The expiry date refers to the last day of the month stated.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Store below 25°C. Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Midanium contains
- The active substance of the medicine is midazolam. 1 ml of the solution contains 1 mg or 5 mg of midazolam.
- The other ingredients are: sodium chloride, disodium edetate, 10% hydrochloric acid, 10% sodium hydroxide or 10% hydrochloric acid (to adjust pH), water for injections.
What Midanium looks like and what the packaging contains
Midanium, 1 mg/ml, solution for injection is a colorless, clear liquid.
The cardboard box contains 10 ampoules of colorless glass, each containing 5 ml.
Midanium, 5 mg/ml, solution for injection is a colorless or light yellow, clear liquid.
The cardboard box contains 10 ampoules of colorless glass, each containing 1 ml or 5 ampoules of colorless glass, each containing 3 ml or 5 ampoules of colorless glass, each containing 10 ml.
Marketing authorization holder and manufacturer
Polpharma S.A.
Pelplińska 19, 83-200 Starogard Gdański
Phone: +48 22 364 61 01
Date of last revision of the leaflet:December 2024
Information intended only for healthcare professionals
MIDANIUM, 1 mg/ml, solution for injection
MIDANIUM, 5 mg/ml, solution for injection
Midazolam
Method of preparation of Midanium for administration and method of administration
- -Midanium is used intramuscularly, intravenously, and rectally.
- -Midazolam should only be administered by experienced doctors in a fully equipped facility with equipment for monitoring and supporting respiratory and cardiovascular functions and by persons properly trained in recognizing and treating expected adverse reactions, including respiratory and cardiac resuscitation.
- -Midanium can be mixed with 500 ml of the following infusion solutions:
- 5% glucose solution;
- 0.9% NaCl solution;
- 4% glucose solution with 0.18% NaCl solution. The prepared solution remains physically and chemically stable during storage at room temperature for 24 hours. From a microbiological point of view, the solution should be used immediately after preparation. If it is not used immediately, the user is responsible for the storage time and conditions before use. It has not been confirmed that midazolam is adsorbed on plastic elements of infusion equipment or syringes.
- Midanium 5 mg/ml can be mixed with morphine sulfate (Morphini sulfas WZF 20 mg/ml) (see also "Warnings and precautions" below) within the following ranges of active substances: 10 mg of morphine sulfate with midazolam in a dose of 1.66 mg to 10 mg. To obtain a mixture of 10 mg of morphine sulfate: 1.66 mg of midazolam, mix 2.1 ml of Morphini sulfas WZF 20 mg/ml with 1.4 ml of Midanium 5 mg/ml. To obtain a mixture of 10 mg of morphine sulfate: 10 mg of midazolam, mix 1.0 ml of Morphini sulfas WZF 20 mg/ml with 4.0 ml of Midanium 5 mg/ml. The shelf life of the mixture of Morphini sulfas WZF 20 mg/ml with Midanium 5 mg/ml - 24 hours. Chemical and physical stability has been demonstrated for 24 hours at 25°C. The mixture should be prepared immediately before administration. From a microbiological point of view, the prepared mixture should be used immediately. If it is not used immediately, the user is responsible for the storage time and conditions. If necessary, the prepared mixture can be stored for up to 24 hours at 25°C, provided that the mixture is prepared in controlled and validated aseptic conditions. Unused mixture within 24 hours should be discarded. The prepared mixture does not require protection from light.
...
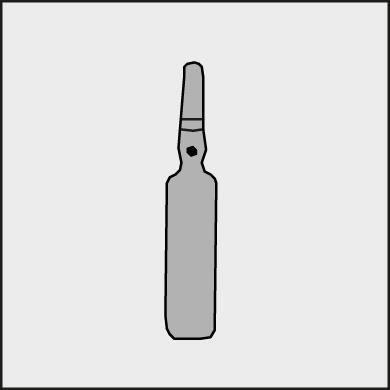
Figure 1.
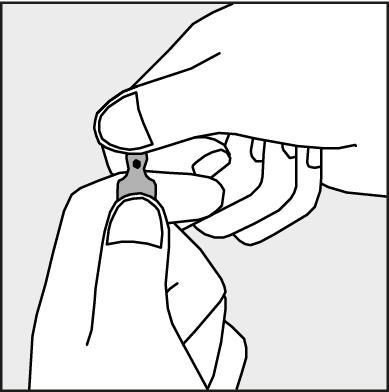
Figure 2.
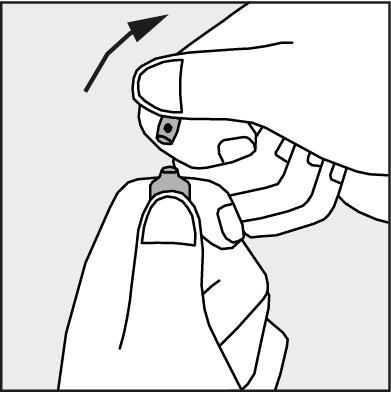
Figure 3.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
A colored dot is placed on each ampoule (see Figure 1), as a mark indicating the break point.
- To open the ampoule, hold it vertically, in both hands, with the colored dot facing you - see Figure 2. Hold the top part of the ampoule in such a way that your thumb is above the colored dot.
- Press according to the arrow on Figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused medicine should be disposed of in accordance with applicable regulations.
...
Children
Neonates and infants up to 6 months
Midazolam should be administered in a continuous intravenous infusion, starting at 0.03 mg/kg/h (0.5 μg/kg/min) in neonates with a gestational age below 32 weeks or 0.06 mg/kg/h (1 μg/kg/min) in neonates with a gestational age above 32 weeks and infants up to 6 months.
It is not recommended to use loading doses in preterm infants, neonates, and infants under 6 months. The drug should be administered by infusion, at a faster rate over the first few hours, to achieve therapeutic serum concentrations. The infusion rate should be carefully and frequently monitored, especially after the first day, to administer the smallest effective dose and prevent potential accumulation of the drug. Close monitoring of respiratory function and oxygen saturation is recommended.
Children over 6 months
In children over 6 months, intubated and mechanically ventilated, a loading dose of 0.05 mg/kg to 0.2 mg/kg should be administered intravenously, slowly over 2 to 3 minutes, to achieve the desired clinical effect.
Midazolam should not be administered by rapid intravenous injection. After the loading dose, a continuous intravenous infusion of 0.06 mg/kg/h to 0.12 mg/kg/h (1 to 2 μg/kg/min) is administered. The infusion rate can be increased or decreased as needed (usually by 25% of the initial or subsequent rate) or additional intravenous doses of midazolam can be administered to increase or maintain the drug's effect at the desired level.
When initiating midazolam administration in patients with impaired cardiovascular function, the drug should be administered in small, consecutive injections, and these patients should be monitored for signs of hemodynamic instability, such as hypotension.
These patients are also prone to the depressant effect of midazolam on the respiratory system and require particularly close monitoring of respiratory function and oxygen saturation.
The use of midazolam solution at concentrations greater than 1 mg/ml is not recommended in preterm infants, neonates, and children with a body weight below 15 kg. Higher concentrations should be diluted to 1 mg/ml.
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MidaniumDosage form: Tablets, 15 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Tablets, 7.5 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Solution, 10 mgActive substance: midazolamManufacturer: MoNo chem-pharm. Produkte GmbHPrescription required
Alternatives to Midanium in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Midanium in Spain
Alternative to Midanium in Ukraine
Online doctors for Midanium
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Midanium – subject to medical assessment and local rules.









