

Maracex

Ask a doctor about a prescription for Maracex

How to use Maracex
Leaflet attached to the packaging: information for the user
Maracex, 20 mg/ml, solution for injection/infusion
Morphine hydrochloride
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor, nurse, or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, nurse, or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Maracex and what is it used for
- 2. Important information before using Maracex
- 3. How to use Maracex
- 4. Possible side effects
- 5. How to store Maracex
- 6. Contents of the packaging and other information
1. What is Maracex and what is it used for
1 ml of the solution contains 20 mg of morphine hydrochloride (hereinafter referred to as morphine). The active substance morphine belongs to a group of medicines called natural opium alkaloids.
This medicine is indicated for the treatment of severe pain that can only be adequately managed with opioid analgesics.
2. Important information before using Maracex
When not to use Maracex:
- if the patient is allergic to morphine hydrochloride or any of the other ingredients of this medicine (listed in section 6);
- if the patient has a large amount of mucus in the airways;
- if the patient has breathing disorders;
- in case of acute liver disease;
- in case of anxiety disorders in patients taking alcohol or sleeping pills.
Warnings and precautions
Before starting treatment with Maracex, you should discuss it with your doctor, nurse, or pharmacist:
- if the patient has impaired lung function (emphysema, heart lung, excessive carbon dioxide in the blood, hypoxia, significant obesity); in such cases, it is particularly important to pay attention to the inhibitory effect of morphine on breathing,
- if the patient has recently had a head injury. The risk of significant increase in intracranial pressure and slowing of breathing in patients with head injuries is higher than usual when using morphine,
- if the patient has asthma or allergies. The effects of morphine releasing histamine may worsen asthma or allergies,
- if the patient regularly consumes alcohol, sleeping pills, or other medicines that affect the central nervous system,
- if the patient has a low blood volume; in this case, the risk of a drop in blood pressure should be considered,
- if the patient has kidney or liver problems,
- in the case of epidural administration, this medicine should be used with caution in patients with a history of neurological disorders or those currently undergoing corticosteroid therapy.
If any of the following symptoms occur during treatment with this medicine, you should consult a doctor, nurse, or pharmacist:
- Increased sensitivity to pain, despite an increase in the dose of the medicine (hyperalgesia). The doctor will decide whether a change in dosage or the use of a stronger painkiller is necessary (see section 2).
- Weakness, fatigue, loss of appetite, nausea, vomiting, or low blood pressure. These may be symptoms that the adrenal glands are producing too little cortisol and hormone supplements may be necessary.
- Loss of sexual desire, impotence, cessation of menstruation. This may be caused by reduced production of sex hormones.
- If the patient has a history of drug or alcohol addiction. You should also tell your doctor if you notice that you are becoming addicted to this medicine as you use it. For example, when you start thinking about taking the next dose frequently, even if you don't need it to relieve pain.
- Withdrawal symptoms or addiction. The most common withdrawal symptoms are listed in section 3. In this case, the doctor may change the medicine or the time between doses.
In the case of long-term treatment administered epidurally, you should immediately inform your doctor if you experience an unexpected increase in pain intensity or any new complaints potentially related to abnormal nervous system function.
Maracex and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
This is especially important when using the following medicines:
- Rifampicin, used to treat, for example, tuberculosis;
- Concomitant use of Maracex and sedative medicines, such as benzodiazepines or derivatives, increases the risk of drowsiness, breathing difficulties (respiratory depression), or coma that can be life-threatening. Therefore, combined treatment should only be considered when other treatment options are not available. If Maracex is used with sedative medicines, the doctor should limit the dose and duration of concomitant use. The patient should tell the doctor about all sedative medicines being taken and strictly follow the prescribed dose.
The effectiveness of treatment may change if this medicine is used with certain other medicines.
Combinations of medicines that should be avoided when using morphine:
- Sedative and sleeping medicines containing barbiturates (methohexital, pentothal, phenobarbital);
- Medicines used to treat depression or Parkinson's disease (MAO inhibitors) (moclobemide, selegiline).
These medicines used in combination with morphine may lead to a decrease in respiratory function.
Medicines that may require dose adjustment:
- Medicines used to treat epilepsy (gabapentin);
- Medicines used to treat depression (clomipramine, amitriptyline, nortriptyline);
- Certain other pain-relieving medicines (buprenorphine, nalbuphine, pentazocine).
Other medicines whose effects may change under the influence of morphine or that may affect its action:
- Muscle relaxants (baclofen);
- Sedative medicines containing benzodiazepines (nitrazepam, flunitrazepam, triazolam, midazolam);
- Sedative medicines that reduce itching (hydroxyzine);
- Stimulants of the central nervous system (methylphenidate);
- Medicines used to treat bleeding in the meninges (nimodipine);
- Medicines used against HIV (ritonavir);
- Certain medicines used to treat blood clots (e.g., clopidogrel, prasugrel, ticagrelor) may have delayed and reduced action when taken with morphine.
Maracex with food, drink, and alcohol
You should avoid combining it with alcohol, as it may lead to worsening of respiratory function.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Pregnancy
Morphine has not been found to cause congenital malformations. Morphine passes through the placenta. Therefore, morphine should only be used during pregnancy if the benefits to the mother clearly outweigh the risks to the child. In the case of labor pain, morphine should only be administered locally into the epidural or spinal space (note: this route of administration of Maracex has not been approved for marketing). If this medicine was taken during pregnancy for a longer period, there is a risk of withdrawal symptoms in the newborn (abstinence syndrome), which should be treated by a doctor.
Breastfeeding
Morphine passes into breast milk, where it reaches a higher concentration than in the mother's plasma. Therefore, this medicine should not be used during breastfeeding.
Fertility
There are no clinical data on the effect of morphine on fertility in men and women.
Driving and using machines
While using this medicine, you should not drive vehicles or operate any tools or machines, as morphine slows down the reaction time and reduces alertness and performance while driving, as well as the precision of performing complex tasks.
Maracex contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per 1 ml, which means that the medicine is considered "sodium-free".
3. How to use Maracex
This medicine should always be used as directed by your doctor. In case of doubts, you should consult your doctor or pharmacist. The dosage is individualized, as the duration of action of morphine and its intensity, the cause and duration of pain are very different, and because morphine is used in very different circumstances. The medicine is usually administered by medical personnel, but in exceptional circumstances (e.g., in pain during palliative care), the patient may also administer the medicine according to the instructions.
This medicine can be administered into a vein (intravenously), into a muscle (intramuscularly), under the skin (subcutaneously), or into the spine (epidurally).
Adults
Subcutaneous or intramuscular administration
Adults: 5 - 20 mg, usually the dose is 10 mg, and it can be repeated every 4 hours if necessary.
Elderly: 5 - 10 mg per dose.
The subcutaneous route is not suitable for patients with edema.
Intravenous administration
Adults: 2.5 - 15 mg (if necessary, diluted in 0.9% sodium chloride solution), administered over 4 to 5 minutes.
Epidural administration
Usually, the initial dose is 2-4 mg, usually diluted in 0.9% sodium chloride solution. After the end of the analgesic effect, usually after 6-24 hours, a new dose of 1-2 mg can be administered if necessary. In the case of long-term pain treatment in cancer patients, usually higher doses and continuous epidural infusion are necessary.
The daily dose usually does not exceed 100 mg per day in adults, but in some individual cases, it may be necessary to use a higher dose to relieve pain, especially in the late stages of the disease.
Elderly patients
The initial dose in elderly patients should be reduced compared to the usual dose, and subsequent dosing should be individualized based on the patient's response. It may also be necessary to reduce the total daily dose if the patient is constantly receiving morphine, as morphine is eliminated more slowly in elderly patients.
Children
Subcutaneous or intramuscular administration:0.1 - 0.2 mg/kg (maximum dose 15 mg). The subcutaneous route of administration is not suitable for patients with edema.
Intravenous administration:0.05 - 0.1 mg/kg, administered very slowly (recommended dilution in 0.9% sodium chloride solution).
Caution should be exercised and a lower dose considered when treating newborns and small children, as they may be sensitive to the effects of opioids, particularly their inhibitory effect on breathing.
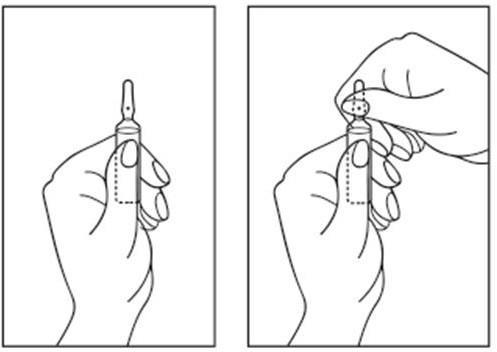
Instructions for opening the ampoule:
- 1) Place the ampoule so that the colored dot is at the top. If there is a part of the solution in the upper part of the ampoule, gently tap with your finger to make the entire solution move to the lower part of the ampoule.
- 2) Use both hands to open; holding the lower part of the ampoule in one hand, break the upper part of the ampoule in the direction opposite to the colored dot (see pictures below).
Using a higher dose of Maracex than recommended
If a higher dose of this medicine is taken than recommended, it may cause pneumonia caused by aspiration of vomit or foreign bodies. Symptoms may include shortness of breath, cough, and fever.
In addition, symptoms of overdose may include breathing difficulties leading to loss of consciousness, and even death.
You should immediately contact the nearest emergency department or a doctor.
Stopping the use of Maracex
You should not stop using this medicine unless your doctor advises you to do so. To stop using this medicine, you should consult your doctor, who will decide how to gradually reduce the dose to avoid withdrawal symptoms. Withdrawal symptoms may include body aches, seizures, diarrhea, stomach pain, nausea, flu-like symptoms, rapid heartbeat, and dilated pupils. Psychological symptoms include intense feelings of dissatisfaction, anxiety, and irritability.
If you have any further doubts about using this medicine, you should consult your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects are fatigue, constipation, nausea, and vomiting, as well as sweating.
You should immediatelycontact your doctor if you experience severe allergic reactions causing breathing difficulties or dizziness.
Other side effects related to the use of this medicine are listed below according to their frequency of occurrence:
Very common (may occur in more than 1 in 10 patients):
Urinary retention after epidural administration.
Common (may occur in up to 1 in 10 patients):
Fatigue, drowsiness, dizziness, constipation, nausea, vomiting, urinary retention after intramuscular or intravenous or subcutaneous administration.
Uncommon (may occur in up to 1 in 100 patients):
Hyperventilation (through the effect on the central nervous system), euphoria, dizziness of labyrinthine origin, headaches, sleep disturbances, anxiety, transient hallucinations, disorientation, balance problems, abnormal vision, increased intracranial pressure, mood changes, excitement, tremors, muscle spasms, seizures, muscle stiffness, dry mouth, psychological and physical dependence, withdrawal symptoms in newborns whose mothers received morphine during pregnancy, such as anxiety, vomiting, increased appetite, irritability, hyperactivity, tremors, or chills, nasal congestion, seizures, crying with high-pitched sounds.
Rare (may occur in up to 1 in 1000 patients):
Slow heartbeat (bradycardia), rapid heartbeat (tachycardia), palpitations, low blood pressure, high blood pressure, facial flushing, hyperventilation, itching, hives, rash, redness, and hardening of the skin at the injection site after intravenous administration.
Very rare (may occur in more than 1 in 10,000 patients):
Bile duct spasm, phlebitis, pulmonary edema, anaphylactic reaction. High doses may cause stimulation of the central nervous system, which may manifest as seizures.
Frequency not known (frequency cannot be estimated from the available data):
Increased sensitivity to pain, sweating, withdrawal symptoms, or addiction (information on symptoms - see section 3: If you stop taking Maracex).
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor, nurse, or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301,
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Maracex
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after: Expiry Date (EXP). The expiry date refers to the last day of the month.
There are no special instructions for the storage temperature of the medicine. Ampoules should be stored in the outer packaging to protect them from light. Do not freeze.
Do not use the medicine containing visible particles.
Conditions for storing the medicine after dilution, see section 6 "Information intended exclusively for healthcare professionals".
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Maracex contains
- The active substance of the medicine is morphine hydrochloride. 1 ml of the solution contains 20 mg of morphine hydrochloride, which corresponds to 15.2 mg of morphine. One ampoule with 1 ml of the solution contains 20 mg of morphine hydrochloride, which corresponds to 15.2 mg of morphine.
- The other ingredients are: sodium chloride, concentrated hydrochloric acid (to adjust pH), water for injections.
What Maracex looks like and what the packaging contains
This medicine is a clear, colorless or slightly yellowish solution for injection/infusion, free from visible particles.
Maracex is available in 1 ml ampoules made of colorless glass type I, in a PVC overwrap, placed in a cardboard box.
Pack size:
10 ampoules of 1 ml
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
AS KALCEKS
Krustpils iela 71E
1057 Rīga
Latvia
Tel.: +371 67083320
Email: [email protected]
Date of last revision of the leaflet: 01/2022 ------------------------------------------------------------------------------------------------------------------------
Information intended exclusively for healthcare professionals:
After opening, the medicine should be used immediately.
For single use only, after use, the remaining contents should be discarded.
Do not use the medicine containing visible particles.
Maracex, 20 mg/ml, solution for injection/infusion should not be mixed with other medicines.
Morphine salts are sensitive to pH changes and may precipitate in an alkaline environment. Incompatible substances with morphine salts are: aminophylline, sodium salts of barbiturates, phenytoin, and ranitidine hydrochloride.
After dilution
Maracex, 20 mg/ml, solution for injection/infusion is compatible with 0.9% sodium chloride solution in a polyethylene container.
Chemical and physical stability has been demonstrated for 28 hours at 25 °C and 2 °C to 8 °C in 0.9% sodium chloride solution in a polyethylene container.
From a microbiological point of view, the diluted solution should be used immediately. If the product is not used immediately, the responsibility for the time and conditions of storage before use, which should not exceed 24 hours at 2 °C to 8 °C, unless the dilution took place in controlled and validated aseptic conditions, rests with the user.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAS Kalceks
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Maracex
Alternatives to Maracex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Maracex in Ukraine
Alternative to Maracex in Spain
Online doctors for Maracex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Maracex – subject to medical assessment and local rules.














