
Autostzhikavka Morfina Pzhecivko Bulovi
Ask a doctor about a prescription for Autostzhikavka Morfina Pzhecivko Bulovi

How to use Autostzhikavka Morfina Pzhecivko Bulovi
Patient Information Leaflet: User Information
AUTO-INJECTOR MORPHINE FOR PAIN RELIEF,
20 mg/2mL, solution for injection
Morphine sulfate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What Auto-Injector Morphine for Pain Relief is and what it is used for
- 2. Important information before using Auto-Injector Morphine for Pain Relief
- 3. How to use Auto-Injector Morphine for Pain Relief
- 4. Possible side effects
- 5. How to store Auto-Injector Morphine for Pain Relief
- 6. Contents of the pack and other information
1. What Auto-Injector Morphine for Pain Relief is and what it is used for
Auto-Injector Morphine for Pain Relief is an auto-injector intended for intramuscular use in case of severe pain caused by extensive bodily injuries. Morphine acts on certain receptors in the central nervous system, causing pain relief.
2. Important information before using Auto-Injector Morphine for Pain Relief
When not to use Auto-Injector Morphine for Pain Relief
- if the patient is undergoing spasmolytic therapy (muscle relaxant therapy),
Consideration of listed contraindications should take into account the acceptable potential risk versus therapeutic benefits in case of severe pain caused by extensive bodily injuries.
Warnings and precautions
Before starting to use Auto-Injector Morphine for Pain Relief, discuss it with your doctor or nurse. Opioid painkillers should be used with caution in patients:
- with reduced thyroid function (hypothyroidism) or Addison's disease (adrenal insufficiency),
- with reduced adrenal cortex function (too little hormone production),
- with asthma or other breathing problems, such as chronic obstructive pulmonary disease (COPD), severe obesity
- with an enlarged prostate or difficulty urinating,
- with low blood pressure,
- with diagnosed heart failure,
- in shock (sudden drop in blood pressure and reduced blood flow through the body's tissues),
- after a severe head injury,
- with a predisposition to seizures,
- with inflammatory bowel disease or impaired bowel patency,
- with muscle weakness (myasthenia),
- abusing drugs,
- with biliary tract disorders (morphine may exacerbate pain),
- with liver function disorders, as morphine metabolism occurs mainly in the liver,
- with kidney function disorders, morphine caused prolonged respiratory depression of severe course,
- over 65 years of age
- debilitated.
Auto-Injector Morphine for Pain Relief should not be used simultaneously with benzodiazepines due to the risk of respiratory depression, coma, and even death. Auto-Injector Morphine for Pain Relief should not be used during poisoning with combat toxic substances belonging to organophosphorus compounds with paralytic-seizure action. The action of organophosphorus compounds and simultaneous administration of morphine may lead to respiratory arrest. In connection with the treatment of Auto-Injector Morphine for Pain Relief, the occurrence of acute generalized exanthematous pustulosis (AGEP) has been reported. Symptoms usually occur within the first 10 days of treatment. You should tell your doctor if you have ever had a severe skin rash or exfoliation after taking Auto-Injector Morphine for Pain Relief or other opioids, or if you have had blisters and (or) ulcers in the mouth. You should stop using Auto-Injector Morphine for Pain Relief and seek medical attention immediately if you notice any of the following symptoms: blisters, widespread exfoliation of the skin, or pustular eruptions with fever. Sleep apnea Auto-Injector Morphine for Pain Relief may cause sleep apnea, such as sleep apnea (pauses in breathing during sleep) and hypoxemia (low oxygen level in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty maintaining sleep, or excessive daytime sleepiness. If you or someone else notices these symptoms, you should contact your doctor. Your doctor may consider reducing the dose of the medicine. You should consult your doctor if you experience severe abdominal pain that may radiate to the back, nausea, vomiting, or fever, as these may be symptoms related to pancreatitis and biliary tract inflammation. If you experience any of the following symptoms while using Auto-Injector Morphine for Pain Relief, you should consult your doctor if:
- you experience increased sensitivity to pain, despite increasing the dose of the medicine (hyperalgesia). Your doctor will decide whether a dose change or the use of a strong painkiller is necessary.
- you experience weakness, fatigue, loss of appetite, nausea, vomiting, or low blood pressure. These may be symptoms that the adrenal glands are producing too little cortisol and hormone supplements may be necessary.
- you experience a loss of sexual desire, impotence, or menstrual cessation. This may be due to reduced production of sex hormones.
- you have a history of drug or alcohol dependence. You should also tell your doctor if you notice that you are becoming dependent on Auto-Injector Morphine for Pain Relief while using it. For example, when you start thinking about taking another dose, even if you don't need it to relieve pain.
- you experience withdrawal symptoms or dependence. The most common withdrawal symptoms are listed in section 3. In this case, your doctor may change the medicine or the time between doses.
Tolerance, dependence, and addictive use This medicine contains morphine, which is an opioid. Repeated use of opioids can lead to reduced effectiveness of the medicine (the patient gets used to it, which is known as tolerance). Repeated use of Auto-Injector Morphine for Pain Relief may also lead to dependence, abuse, and addictive use, which can result in life-threatening overdose. The risk of these adverse reactions may increase with increasing dose and duration of use. Dependence or addictive use can cause the patient to feel a loss of control over how much medicine to take or how often to take it. The risk of dependence or addictive use varies from person to person. The risk of dependence on Auto-Injector Morphine for Pain Relief or its addictive use may be higher if:
- the patient or any of their relatives have ever abused or been dependent on alcohol, prescription drugs, or illicit drugs ("addiction");
- the patient smokes;
- the patient has ever had mood disorders (depression, anxiety disorders, or personality disorders) or has been treated by a psychiatrist for other mental illnesses.
If you experience any of the above symptoms while taking Auto-Injector Morphine for Pain Relief, it may indicate dependence or addictive use:
- you need to take the medicine for a longer period than prescribed by your doctor;
- you need to take a higher dose than prescribed;
- you use the medicine for reasons other than those for which the doctor prescribed it, for example, "to calm down" or "to fall asleep";
- you have repeatedly tried to stop or control the use of the medicine;
and
- if you stop taking the medicine, you feel unwell, and your condition improves when you take the medicine again ("withdrawal effect").
If you notice any of these symptoms, you should discuss with your doctor the best treatment strategy for you, including when it is appropriate to stop the treatment and how it can be safely terminated (see section 3 "Stopping the use of Auto-Injector Morphine for Pain Relief"). When administering morphine during the pre-, peri-, and postoperative period, one should be aware of the risk of paralytic ileus or respiratory depression. The analgesic effect of morphine may mask many intra-abdominal complications, such as intestinal perforation.
Children and adolescents
The medicine is not intended for use in children and adolescents.
Auto-Injector Morphine for Pain Relief with other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take. This is especially important for medicines such as:
- monoamine oxidase inhibitors (antidepressants and for the treatment of Parkinson's disease), such as moclobemide, especially if you have taken them in the last 2 weeks. Do not use these medicines with morphine - see section 2, subsection: "When not to use Auto-Injector Morphine for Pain Relief",
- general anesthetics,
- sleeping pills,
- tranquilizers, anxiolytics, such as diazepam
- psychotropic drugs (used to treat depression, psychosis, neurosis),
- antihistamines (anti-allergic drugs),
- beta-blockers (drugs used to treat high blood pressure, coronary heart disease, arrhythmias), other drugs used to treat high blood pressure,
- phenothiazine derivatives (antipsychotic drugs),
- muscle relaxants and heart rate regulators, such as atropine
- ciprofloxacin
- drugs that accelerate the passage of food through the digestive tract, such as cisapride, anti-emetics (metoclopramide, domperidone);
- dopamine-ergic drugs, such as those used in Parkinson's disease, selegiline;
- mexiletine (a drug for arrhythmias);
- cimetidine (a drug for stomach or duodenal ulcers)
- rifampicin (used to treat tuberculosis)
The sedative effect of morphine on the central nervous system is enhanced by substances such as alcohol, anesthetics, sleeping pills, tranquilizers, anxiolytics, tricyclic antidepressants, antihistamines, beta-blockers, and phenothiazine derivatives. Concurrent use of Auto-Injector Morphine for Pain Relief and tranquilizers, such as benzodiazepines or derivatives, increases the risk of drowsiness, breathing difficulties (respiratory depression), or coma, which can be life-threatening. Therefore, do not use them together without consulting your doctor. Combination therapy may be considered by your doctor only when other treatment options are not available. If Auto-Injector Morphine for Pain Relief is used together with tranquilizers, your doctor should limit the dose of the medicine and the duration of concurrent use. You should tell your doctor about all tranquilizers you are taking and strictly follow the dose prescribed by your doctor. It may be helpful to inform a relative or close friend about the possibility of the above symptoms. If these symptoms occur, you should consult your doctor. Some medicines used to treat blood clots (e.g., clopidogrel, prasugrel, ticagrelor) may have delayed and reduced effects when taken with morphine. Many medicines can interact with morphine sulfate for injection, which can significantly alter their effects. These include:
- Gabapentin or pregabalin used to treat epilepsy and pain caused by nervous system disorders (neuropathic pain).
Auto-Injector Morphine for Pain Relief with alcohol
Do not drink alcohol while using morphine, as it may enhance the sedative effect of morphine on the central nervous system (including the respiratory center).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor for advice before taking this medicine. Pregnancy In women of childbearing potential, the doctor may use morphine only when absolutely necessary, when the potential risk is acceptable in view of the expected therapeutic benefits. Morphine should not be used during labor. If morphine was taken during pregnancy for a longer period, there is a risk of withdrawal symptoms in the newborn, which should be treated by a doctor. Breastfeeding Morphine passes into breast milk. If the doctor prescribes morphine, breastfeeding should be stopped. Fertility In animal studies, it has been shown that morphine can reduce fertility.
Driving and using machines
Morphine can cause drowsiness and impair psychophysical performance. While using the medicine, do not drive vehicles or operate machines.
Auto-Injector Morphine for Pain Relief contains sodium metabisulfite
The medicine may rarely cause severe hypersensitivity reactions and bronchospasm, which can lead to breathing difficulties.
Auto-Injector Morphine for Pain Relief contains sodium
1 mL of Auto-Injector Morphine for Pain Relief contains 3.4 mg of sodium, i.e., an auto-injector with 2 mL of solution contains less than 23 mg of sodium per dose, which means that the medicine is considered "sodium-free".
3. How to use Auto-Injector Morphine for Pain Relief
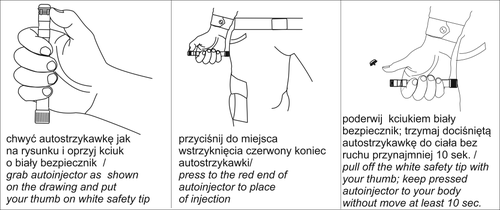
The medicine should be used in case of severe pain caused by extensive bodily injuries. Auto-Injector Morphine for Pain Relief provides a single dose of 20 mg in intramuscular administration. The medicine is intended for single use. The administered dose is sufficient to ensure analgesic effect in an adult patient weighing about 70 kg. The analgesic effect is ensured within 15 to 20 minutes, and the full effect should be achieved within 1 hour after administration. A single dose is sufficient for most patients of standard height. Before starting and regularly during treatment, your doctor will discuss with you what to expect from using Auto-Injector Morphine for Pain Relief, when and for how long to take it, when to consult a doctor, and when to stop using the medicine (see also "Stopping the use of Auto-Injector Morphine for Pain Relief" in this section). Auto-Injector Morphine for Pain Relief should be: removed from the outer packaging (polyethylene tube - to open the tube, unscrew the cap, breaking the seal), press the red end of the auto-injector against the injection site, lift the white safety guard, and hold it against the body without moving for 10 seconds. Follow the instructions on the diagram:
Using a higher than recommended dose of Auto-Injector Morphine for Pain Relief
In case of morphine overdose, the following symptoms are observed: pinpoint pupils, breathing difficulties, low blood pressure (hypotension), increased heart rate, dizziness, feeling cold (hypothermia), and muscle relaxation. In severe cases, the following may occur: low blood pressure causing difficulties in pumping blood through the heart to the entire body (circulatory failure), deepening coma, rapid breakdown of muscle tissue (characterized by darkening of urine and tenderness, stiffness, and pain in the muscles), leading to kidney failure. If a higher than recommended dose of Auto-Injector Morphine for Pain Relief is taken, respiratory depression, coma, or even death may occur. Additionally, overdose symptoms may include breathing difficulties leading to loss of consciousness and even death.
Stopping the use of Auto-Injector Morphine for Pain Relief
Withdrawal symptoms may include body aches, seizures, diarrhea, stomach pain, flu-like symptoms, rapid heartbeat, and dilated pupils. Psychological symptoms include intense dissatisfaction, anxiety, and irritability.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. You should contact your doctor immediately if you experience any of the serious side effects listed below.
- severe allergic reactions after intravenous administration of morphine (rare - less than 1 in 1,000 patients) and anaphylactoid reactions (frequency not known) causing hives, itching, swelling of the face, lips, tongue, throat, causing difficulty breathing or swallowing, dizziness, loss of consciousness
- severe skin reaction with blisters, widespread exfoliation of the skin, pustular eruptions with fever. This may be a condition called acute generalized exanthematous pustulosis (AGEP).
- breathing difficulties leading to loss of consciousness
- withdrawal symptoms or dependence (symptoms are described in section 3: "Stopping the use of Auto-Injector Morphine for Pain Relief").
During treatment with morphine, the following side effects may also be observed with unknown frequency (cannot be estimated from the available data): anxiety, low mood, hallucinations, drowsiness, confusion, increased intracranial pressure, dizziness, unpleasant sensations due to stimulation, which in healthy people does not cause pain, hyperalgesia, excessive sweating, miosis, bradycardia, palpitations, hypotension, orthostatic hypotension, dry mouth, constipation, nausea, vomiting, spasm of the biliary tract, hives, itching, muscle stiffness, difficulty urinating, spasm of the ureters, antidiuretic effect (inhibition of water excretion in the urine), flushing, sweating, contact dermatitis, pain and irritation at the injection site, as well as: sleep apnea (moments of breathing cessation during sleep), symptoms related to pancreatitis and biliary tract inflammation, such as severe abdominal pain that may radiate to the back, nausea, vomiting, or fever.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. Side effects can be reported to the Department of Monitoring of Adverse Reactions to Medicinal Products, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warszawa, tel. (22) 49 21 301, fax (22) 49 21 309, website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Auto-Injector Morphine for Pain Relief
Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the label after EXP. The expiry date refers to the last day of that month. The batch number (Lot) on the packaging indicates the batch number. Store in a temperature below 25°C. Do not freeze.
6. Contents of the pack and other information
What Auto-Injector Morphine for Pain Relief contains
The active substance of the medicine is morphine sulfate. 2 mL of solution for injection contains 20 mg of morphine sulfate. The other ingredients are: sodium chloride, sodium metabisulfite (E 223), disodium edetate, water for injections
What Auto-Injector Morphine for Pain Relief looks like and what the pack contains
An auto-injector with a single-chamber cartridge made of PP with a clear, colorless or light yellow solution, closed on one side with a rubber stopper, on the other side with a rubber plunger, and a steel needle inserted inside. The auto-injector is placed in a semi-transparent polyethylene tube closed with a breakable cap with a safety seal. The packaging contains 1 auto-injector with 20 mg of morphine sulfate in 2 mL of solution.
Marketing authorization holder and manufacturer
Ravimed Sp. z o.o. ul. Polna 54 05-119 Łajski Tel.: +48 22 782-21-67
Date of last revision of the leaflet:
Information intended for healthcare professionals only:
Morphine salts have been shown to be physically incompatible with the following medicinal substances: sodium acyclovir, aminophylline (theophylline-ethylenediamine), sodium amobarbital, cefepime hydrochloride, sodium chlorothiazide, sodium floxacin, furosemide, sodium heparin, meperidine hydrochloride, sodium methicillin, minocycline hydrochloride, gallium nitrate, sodium phenobarbital, sodium phenytoin, sargramostim, sodium bicarbonate, sodium thiopental. Physical incompatibility (precipitation) has been demonstrated between morphine sulfate solutions and 5-fluorouracil solutions.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakład Produkcji Sprzętu Medycznego RAVIMED Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Autostzhikavka Morfina Pzhecivko BuloviDosage form: Solution, 20 mg/mlActive substance: morphineManufacturer: AS KalceksPrescription requiredDosage form: Solution, 10 mg/mlActive substance: morphinePrescription requiredDosage form: Solution, 1 mg/mlActive substance: morphinePrescription not required
Alternatives to Autostzhikavka Morfina Pzhecivko Bulovi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Autostzhikavka Morfina Pzhecivko Bulovi in Ukraine
Alternative to Autostzhikavka Morfina Pzhecivko Bulovi in Spain
Online doctors for Autostzhikavka Morfina Pzhecivko Bulovi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Autostzhikavka Morfina Pzhecivko Bulovi – subject to medical assessment and local rules.














