
Lungamo
Ask a doctor about a prescription for Lungamo

How to use Lungamo
Leaflet accompanying the packaging: information for the user
Lungamo, 18 micrograms/dose, powder for inhalation in a capsule
Hard
Tiotropium
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- -You should keep this leaflet, so that you can read it again if necessary.
- -You should consult a doctor or pharmacist if you have any doubts.
- -This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- -If you experience any side effects, including any possible side effects not listed in the leaflet, you should tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Lungamo and what is it used for
- 2. Important information before using Lungamo
- 3. How to use Lungamo
- 4. Possible side effects
- 5. How to store Lungamo
- 6. Contents of the packaging and other information
1. What is Lungamo and what is it used for
Lungamo contains the active substance tiotropium. Lungamo makes it easier for people with chronic obstructive pulmonary disease (COPD) to breathe. COPD is a chronic lung disease that causes shortness of breath and coughing. The name COPD is associated with chronic bronchitis and emphysema. COPD is a chronic disease, so Lungamo should be taken every day, and not just when breathing problems or other COPD symptoms occur. Lungamo is a long-acting bronchodilator that helps to expand the airways and make it easier for air to enter and leave the lungs. Regular use of Lungamo can also help reduce persistent shortness of breath associated with the disease and help reduce the impact of the disease on daily life. Daily use of Lungamo will also help prevent sudden, short-term worsening of COPD symptoms, which can last for several days.
2. Important information before using Lungamo
When not to use Lungamo
- If the patient is allergic to tiotropium or any of the other ingredients of this medicine (listed in section 6),
- If the patient is allergic to atropine or its derivatives, such as ipratropium or oxytropium.
Warnings and precautions
Before starting to use Lungamo, you should discuss it with your doctor or pharmacist.
- The patient should contact their doctor if they have narrow-angle glaucoma, prostate problems, or difficulty urinating.
- The patient should contact their doctor if they have kidney function disorders.
- Lungamo is indicated for the treatment of chronic obstructive pulmonary disease. It should not be used to treat sudden attacks of shortness of breath or wheezing.
- During the use of Lungamo, immediate hypersensitivity reactions, such as rash, swelling, itching, wheezing, or shortness of breath, may occur. In such cases, you should contact your doctor immediately.
- Medicines used by inhalation, such as Lungamo, may cause chest tightness, coughing, wheezing, or shortness of breath immediately after use. In such cases, you should contact your doctor immediately.
- Caution should be exercised to avoid getting the inhalation powder into the eyes, as it may cause the occurrence or exacerbation of glaucoma symptoms, which is an eye disease. Eye pain or discomfort, blurred vision, seeing a rainbow-colored ring around a light source, or changed color vision, along with eye redness, may be a sign of acute glaucoma. Eye disorders may be accompanied by: headache, nausea, and vomiting. If symptoms of glaucoma occur, you should stop using Lungamo and contact your doctor immediately, preferably an ophthalmologist.
- Dryness of the mouth, which has been observed during anticholinergic treatment, may cause tooth decay over a longer period. Therefore, it is essential to maintain good oral hygiene.
- If the patient has had a heart attack in the last 6 months or has had unstable or life-threatening heart rhythm disorders in the last year, they should inform their doctor. It is crucial to make an appropriate decision about whether Lungamo can be used in the patient.
- This medicine should not be used more than once a day.
Children and adolescents
Lungamo is not recommended for children and adolescents under 18 years of age.
Lungamo and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as those that are available without a prescription. In particular, you should inform your doctor or pharmacist about other medicines used to treat lung disease, such as ipratropium or oxytropium. No interactions have been reported when taking Lungamo with other medicines used to treat COPD, such as inhaled rescue medicines, e.g., salbutamol, methylxanthines, e.g., theophylline, and oral or inhaled steroids, e.g., prednisolone.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine. You should not use this medicine unless your doctor has prescribed it.
Driving and using machines
Dizziness, blurred vision, or headache may affect your ability to drive or use machines.
Lungamo contains lactose
If you have previously been diagnosed with an intolerance to some sugars, you should contact your doctor before taking this medicine. Lactose contains small amounts of proteins that may cause allergic reactions.
3. How to use Lungamo
Lungamo should always be used as directed by your doctor. If you have any doubts, you should contact your doctor or pharmacist. It is recommended to inhale the contents of one capsule (18 micrograms of tiotropium) once a day. You should perform2 inhalations from the same capsule(see the "Instructions for use and maintenance" section at the end of this leaflet). You should not use a higher dose than recommended. You should take the capsule at the same time every day. This is important because Lungamo works for 24 hours.
Use in children and adolescents
Lungamo is not recommended for children and adolescents under 18 years of age.
Method of administration
The capsules can only be used for inhalation and should not be taken orally. The capsules should not be swallowed. The capsule should be removed from the blister pack immediately before using the inhaler! The Vertical-Haler inhaler, into which the Lungamo capsule is inserted, pierces the capsule and allows the powder to be inhaled. The patient should ensure that they have a Vertical-Haler inhaler and use it correctly. The instructions for using the Vertical-Haler inhaler are at the end of this leaflet. If you feel that breathing difficulties are worsening, you should contact your doctor as soon as possible.
Using a higher dose of Lungamo than recommended
If you use more than one Lungamo capsule per day, you should contact your doctor immediately. The risk of side effects, such as dryness of the mouth, constipation, difficulty urinating, rapid heartbeat, or blurred vision, may increase in the patient.
Missing a dose of Lungamo
If you miss a dose, you should take it as soon as possible, but never take two doses at the same time or on the same day. The next dose should be taken as usual. You should not take a double dose to make up for a missed dose.
Stopping the use of Lungamo
Before stopping the use of Lungamo, you should consult your doctor or pharmacist. After stopping the use of this medicine, COPD symptoms may worsen. If you have any further doubts about using this medicine, you should contact your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects have been reported by patients using this medicine. Severe side effects, such as allergic reactions, which can cause swelling of the faceor throat(angioedema), or other hypersensitivity reactions(such as a sudden drop in blood pressure or dizziness) may occur individually or as part of a severe allergic reaction (anaphylactic reaction) after using Lungamo. Additionally, some patients may experience: unexpected chest tightness, coughing, wheezing, or shortness of breathimmediately after inhaling the medicine (bronchospasm).
If you experience any of these symptoms, you should contact your
doctorimmediately.
Other side effects
Common (may affect up to 1 in 10 people)
- Dryness of the mouth
Uncommon (may affect up to 1 in 100 people)
- Dizziness
- Headache
- Taste disorders
- Blurred vision
- Irrregular heartbeat (atrial fibrillation)
- Pharyngitis
- Hoarseness (dysphonia)
- Coughing
- Heartburn (gastroesophageal reflux disease)
- Constipation
- Fungal infections of the mouth and throat (oral and pharyngeal candidiasis)
- Rash
- Difficulty urinating (urinary retention)
- Painful urination (dysuria)
Rare (may affect up to 1 in 1000 people)
- Difficulty sleeping (insomnia)
- Seeing a rainbow-colored ring around a light source or changed color vision, along with eye redness (glaucoma)
- Increased eye pressure
- Irrregular heartbeat (supraventricular tachycardia)
- Rapid heartbeat (tachycardia)
- Feeling of rapid heartbeat (palpitations)
- Chest tightness associated with coughing, wheezing, or shortness of breath occurring immediately after inhaling the medicine (bronchospasm)
- Nosebleeds
- Laryngitis
- Sinusitis
- Intestinal obstruction or lack of bowel movements (intestinal obstruction, including paralytic ileus)
- Gingivitis
- Glossitis
- Difficulty swallowing (dysphagia)
- Stomatitis
- Nausea
- Hypersensitivity, including immediate reactions
- Severe allergic reaction, which can cause swelling of the face or throat (angioedema)
- Hives (urticaria)
- Itching (pruritus)
- Urinary tract infections
Frequency not known (frequency cannot be estimated from the available data)
- Dehydration
- Tooth decay
- Severe allergic reactions (anaphylactic reactions)
- Skin infections or ulcers
- Dry skin
- Joint swelling
Reporting side effects
If you experience any side effects, including any possible side effects not listed in the leaflet, you should tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Lungamo
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date (EXP) stated on the carton and blister pack. The expiry date refers to the last day of that month. Do not store above 30°C. The inhaler should be discarded after 90 days of first use. The capsule should be used immediately after opening the blister pack. Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Lungamo contains
The active substance is tiotropium. Each capsule contains 22.5 micrograms of tiotropium bromide monohydrate, which corresponds to 18 micrograms of tiotropium. The delivered dose (released from the Vertical-Haler mouthpiece) is 10 micrograms of tiotropium. The medicine also contains lactose monohydrate (which may contain small amounts of milk proteins). The capsule shell contains gelatin, purified water, macrogol 4000, titanium dioxide (E171), yellow iron oxide (E172), and brilliant blue (E133).
What Lungamo looks like and contents of the pack
Lungamo is a non-transparent green capsule with a size of 16 mm x 5.8 mm containing a powder for inhalation. The blister pack contains 5 or 10 capsules. Lungamo is available in the following packs:
- Carton containing a Vertical-Haler inhaler and 10 capsules
- Carton containing a Vertical-Haler inhaler and 15 capsules
- Carton containing a Vertical-Haler inhaler and 30 capsules
- Carton containing a Vertical-Haler inhaler and 60 capsules
- Carton containing a Vertical-Haler inhaler and 90 capsules
- Hospital pack: Carton containing a Vertical-Haler inhaler and 5x30 capsules (bulk pack)
- Carton containing two Vertical-Haler inhalers and 60 capsules
- Carton containing three Vertical-Haler inhalers and 90 capsules
- Hospital pack: Carton containing 5x60 capsules (bulk pack)
- Carton containing 30 capsules
- Carton containing 60 capsules
- Carton containing 90 capsules
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
STADA Arzneimittel AG,
Stadastrasse 2-18,
61118 Bad Vilbel,
Germany
Manufacturer/Importer
Pharmadox Healthcare Limited
KW20A Kordin Industrial Park,
Paola, PLA 3000
Malta
STADA Arzneimittel AG
Stadastrase 2-18
61118 Bad Vilbel
Germany
To obtain more detailed information, you should contact the representative of the marketing authorization holder.
STADA Pharm Sp. z o.o.
ul. Krakowiaków 44
02-255 Warsaw
tel. +48 22 737 79 20
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany
Tiotropium STADA AG 18 Mikrogramm Hartkapseln mit Pulver zur Inhalation
Date of last revision of the leaflet:
Instructions for use and maintenance
You should carefully follow your doctor's instructions for using Lungamo. The Vertical-Haler inhaler has been specifically designed for Lungamo. You should not use it to take any other medicines. The Vertical-Haler inhaler can be used to take the medicine for a period of up to 90 days.
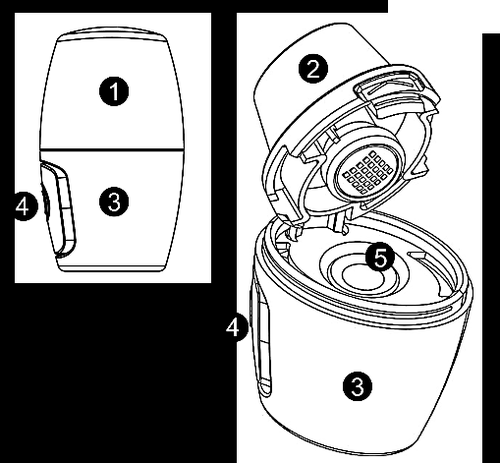 | Vertical-Haler inhaler – explanation of individual parts 1 Cover 2 Mouthpiece 3 Casing 4 Button 5 Capsule chamber |
| |
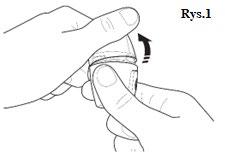 | |
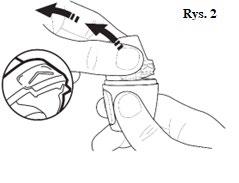 |
|
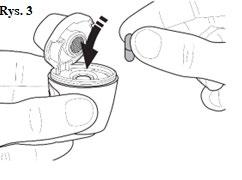 |
|
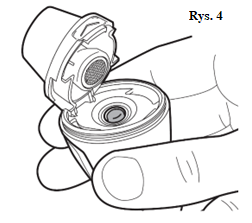 | You should ensure that the capsule is properly inserted (Fig. 4). |
| |
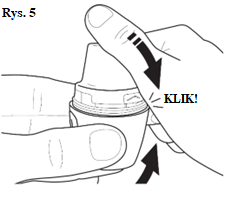 | |
| |
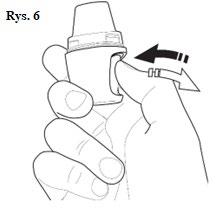 | |
| |
 |
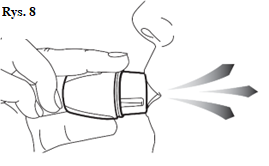 |
|
| Note: While holding the inhaler during inhalation, be careful not to block the holes on the sides of the mouthpiece. This may make it difficult for air to flow inside the inhaler, reducing the movement of the capsule and the dispersion of the medicine. Do not press the mouthpiece during inhalation. This may block the movement of the capsule. | |
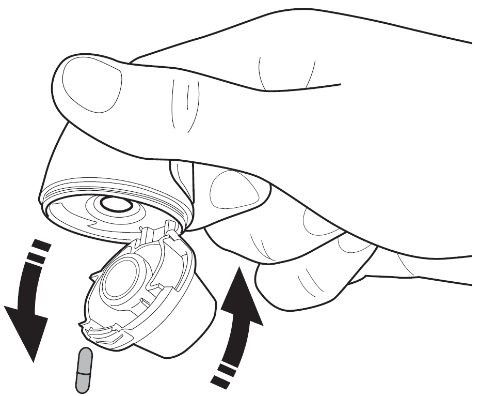 | Cleaning the Vertical-Haler inhaler After use, open the mouthpiece. Hold the inhaler with the opening facing down to remove the capsule from the capsule chamber. Clean the mouthpiece and capsule chamber with a clean, dry cloth. If necessary, you can use a soft, clean brush to remove any remaining powder from the chamber. Close the mouthpiece and put on the cover. This will help keep the inhaler clean and free of dust. If necessary, the outer part of the mouthpiece can be cleaned with a damp cloth. |
Note:
The Lungamo capsule contains only a small amount of powder for inhalation, so it is only partially filled.
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterPharmadox Healthcare Limited STADA Arzneimittel AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LungamoDosage form: Powder, 18 mcgActive substance: tiotropium bromideManufacturer: Ferrer Internacional, S.A.Prescription requiredDosage form: Powder, 10 mcgActive substance: tiotropium bromideManufacturer: Actavis Ltd. Laboratorios Liconsa S.A. Teva Operations Poland Sp. z o.o. Teva Pharma B.V.Prescription requiredDosage form: Powder, 18 mcg/measured doseActive substance: tiotropium bromidePrescription required
Alternatives to Lungamo in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lungamo in Ukraine
Alternative to Lungamo in Spain
Online doctors for Lungamo
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lungamo – subject to medical assessment and local rules.














