
How to use Kliogest
PATIENT INFORMATION LEAFLET
Enclosed leaflet: information for the user
Kliogest, 2 mg + 1 mg, coated tablets
estradiol + norethisterone acetate
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What Kliogest is and what it is used for
- 2. Important information before taking Kliogest
- 3. How to take Kliogest
- 4. Possible side effects
- 5. How to store Kliogest
- 6. Contents of the pack and other information
1. What Kliogest is and what it is used for
Kliogest is a medicine for continuous combined hormone replacement therapy (HRT), taken daily without interruption. Kliogest is used in postmenopausal women, at least one year after their last natural menstrual period.
The tablets contain two hormones: estradiol 2 mg (estrogen, identical to that produced by the ovaries in women) and norethisterone acetate 1 mg (progestogen, acting similarly to the progesterone hormone produced in the human body).
Kliogest is used to:
relieve symptoms occurring after menopause
During menopause, the amount of estrogen produced in a woman's body decreases, which can cause symptoms such as a feeling of heat on the face, neck, and chest ("hot flashes").
Kliogest relieves these symptoms after menopause. Kliogest may be prescribed to a patient only if the symptoms significantly impair daily life;
prevent osteoporosis
After menopause, some women develop brittle bones (osteoporosis). All available options should be discussed with a doctor.
If a patient is at increased risk of fractures due to osteoporosis and other medicines are not suitable, Kliogest can be used to prevent osteoporosis after menopause.
Kliogest is recommended for women with an intact uterus and who have been amenorrheic for at least one year.
Experience in treating women over 65 years of age is limited.
2. Important information before taking Kliogest
Medical history and regular medical examinations
Taking HRT involves risks that should be considered when a patient decides to take hormonal replacement therapy or continue it.
Experience in treating women in premature menopause (due to ovarian failure or surgical procedure) is limited. If a patient is experiencing premature menopause, the risk associated with HRT may differ. Consult a doctor.
Before starting (or resuming) HRT, the doctor should take a medical history, including a family history. The doctor may decide to perform tests, including a breast examination and/or gynecological examination, if necessary.
If a patient starts taking Kliogest, she should regularly visit her doctor for check-ups (at least once a year). During these check-ups, she should discuss the benefits and risks of continuing Kliogest with her doctor.
The patient should regularly undergo breast examinations, as recommended by her doctor.
When not to take Kliogest
In case of any of the following diseases or in case of doubt, inform your doctorbefore taking Kliogest.
Do not start taking Kliogest if:
- the patient is allergic(hypersensitive) to estradiol, norethisterone acetateor any of the other ingredients of Kliogest (listed in section 6. Contents of the pack and other information);
- breast cancer has been diagnosed, has occurred in the past, or is suspected;
- endometrial cancer (cancer of the uterine lining) or other estrogen-dependent tumors have been diagnosed, have occurred in the past, or are suspected;
- there is unexplained vaginal bleeding;
- there is endometrial hyperplasia(excessive thickening of the uterine lining) and it is not being treated;
- blood clots in the veins (venous thromboembolism), e.g., deep vein thrombosis or pulmonary embolism, have been diagnosed or have occurred in the past;
- there is a blood clotting disorder(such as protein C, protein S, or antithrombin deficiency);
- there are or have been diseases caused by blood clots in the arteries, such as heart attack, stroke, or angina;
- there is or has been liver disease, and liver function tests have not returned to normal;
- there is a rare, inherited blood disorder, porphyria.
Warnings and precautions
Before starting treatment, inform your doctor if you have had any of the following diseases, as they may recur or worsen during Kliogest treatment. In such cases, your doctor may require more frequent monitoring:
- uterine fibroids (fibroids),
- endometriosis (growth of uterine lining outside the uterus) or endometrial hyperplasia (excessive thickening of the uterine lining) in the past,
- factors that increase the risk of blood clots (see Blood clots in the veins (venous thromboembolism)),
- factors that increase the risk of estrogen-dependent tumors (such as breast cancer in the patient's mother, sister, or grandmother),
- high blood pressure,
- liver disease, such as a benign liver tumor,
- diabetes,
- gallstones,
- migraine or severe headaches,
- systemic lupus erythematosus (a disease that affects many organs),
- epilepsy,
- asthma,
- otosclerosis (a disease that leads to gradual hearing loss),
- high levels of fats (triglycerides) in the blood,
- fluid retention due to heart or kidney problems,
- a condition in which the thyroid gland does not produce enough thyroid hormones (hypothyroidism) and thyroid replacement therapy is being taken,
- a hereditary tendency to recurrent episodes of severe swelling (hereditary angioedema) or if swelling of the hands, face, feet, mouth, eyes, tongue, or throat (airway obstruction) or gastrointestinal tract (acquired angioedema) has occurred,
- lactose intolerance.
If any of the following diseases occur during HRT, stop taking Kliogest and consult your doctor immediately:
- if any of the diseases listed in the When not to take Kliogestsection occur,
- if the skin or whites of the eyes turn yellow (jaundice), which may be a sign of liver dysfunction,
- if facial, tongue, and/or throat swelling occurs, and/or difficulty swallowing or hives, which may indicate angioedema,
- if there is a significant increase in blood pressure (symptoms may include headache, fatigue, and dizziness),
- if a migraine-type headache occurs for the first time,
- if pregnancy is confirmed,
- if symptoms of a blood clot occur, such as:
- painful swelling and redness of the legs,
- sudden chest pain,
- difficulty breathing. For more information, see Blood clots in the veins (venous thromboembolism).
Note:Kliogest is not a contraceptive. If it has been less than 12 months since the last menstrual period or the patient is under 50 years of age, an additional method of contraception may be necessary. Consult a doctor.
HRT and cancer
Endometrial hyperplasia (excessive thickening of the uterine lining) and endometrial cancer
Taking only estrogens in HRT increases the risk of endometrial hyperplasia and endometrial cancer.
The progestogen in Kliogest helps reduce this additional risk.
Comparison
In women aged 50-65 with an intact uterus who do not take HRT, endometrial cancer is diagnosed in approximately 5 out of 1000 women.
In women aged 50-65 with an intact uterus who take only estrogens in HRT, the number of cases of endometrial cancer diagnosed depends on the duration of treatment and the dose of estrogens taken, ranging from 10 to 60 out of 1000 women (i.e., 5 to 55 additional cases).
Unexpected bleeding
During the first 3 to 6 months of taking Kliogest, unexpected bleeding or spotting may occur. However, if unexpected bleeding:
- persists for more than the first 6 months,
- occurs after taking Kliogest for more than 6 months,
- persists after stopping Kliogest, consult your doctor as soon as possible.
Breast cancer
Data confirm that taking HRT in the form of combined estrogen and progestogen or estrogen alone increases the risk of breast cancer. The additional risk depends on the duration of HRT. This additional risk becomes apparent after 3 years of HRT. After stopping HRT, the additional risk will decrease over time, but the risk may persist for 10 years or more if HRT lasted more than 5 years.
Comparison
In women aged 50-54 who do not take HRT, breast cancer is diagnosed in approximately 13 to 17 out of 1000 women over a 5-year period.
In women aged 50 who start a 5-year estrogen-only HRT, the number of cases will be 16-17 out of 1000 patients (i.e., 0 to 3 additional cases).
In women aged 50 who start a 5-year combined estrogen-progestogen HRT, the number of cases will be 21 out of 1000 patients (i.e., 4 to 8 additional cases).
In women aged 50-59 who do not take HRT, breast cancer is diagnosed in approximately 27 out of 1000 women over a 10-year period.
In women aged 50 who start a 10-year estrogen-only HRT, the number of cases will be 34 out of 1000 patients (i.e., 7 additional cases).
In women aged 50 who start a 10-year combined estrogen-progestogen HRT, the number of cases will be 48 out of 1000 patients (i.e., 21 additional cases).
Regular breast examinations should be performed. Consult your doctor if you notice any of the following changes:
- skin retraction,
- nipple changes,
- lumps that are visible or palpable.
In addition, it is recommended to perform screening mammograms as advised by your doctor. Before the examination, inform the nurse or medical staff performing the X-ray that you are taking HRT, as this medication may increase breast density, which can affect the mammogram result. Not all lumps can be detected during a mammogram in areas with increased breast density.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. Taking HRT with only estrogens or combined estrogen and progestogen is associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer depends on age. For example, in women aged 50-54 who do not take HRT, ovarian cancer is diagnosed over a 5-year period in approximately 2 out of 2000 women.
In women who took HRT for 5 years, the number of cases is approximately 3 out of 2000 (i.e., 1 additional case).
Effect of HRT on the heart and circulation
Blood clots in the veins (venous thromboembolism)
The risk of blood clots in the veinsis 1.3 to 3 times higher in women taking HRT compared to those not taking HRT, especially in the first year of treatment.
Blood clots can be life-threatening and, if they move to the lungs, can cause chest pain, shortness of breath, loss of consciousness, or even death.
The risk of blood clots in the veins is higher if the patient is older and if any of the following factors occur. Inform your doctor if:
- the patient is unable to walk for an extended period due to major surgery, injury, or illness (see 3. If surgery is planned);
- obesity is present (body mass index - BMI > 30 kg/m²);
- there are blood clotting disorders that require long-term anticoagulant therapy;
- anyone in the patient's immediate family has had blood clots in the legs, lungs, or other organs;
- systemic lupus erythematosus is present;
- the patient has been diagnosed with cancer.
If symptoms of a blood clot occur, see If any of the following diseases occur during HRT, stop taking Kliogest and consult your doctor immediately.
Comparison
In women aged 50-59 who do not take HRT, the estimated number of cases of blood clots in the veins over a 5-year period is 4 to 7 out of 1000 women.
In women aged 50-59 who take combined estrogen-progestogen HRT, the number of cases over a 5-year period will be 9 to 12 out of 1000 women (i.e., 5 additional cases).
Coronary artery disease (heart attack)
There is no evidence that HRT helps prevent heart attacks. In women over 60 years of age taking combined estrogen-progestogen HRT, there is a slightly increased tendency to develop coronary artery disease compared to women not taking HRT.
Stroke
The risk of stroke is approximately 1.5 times higher in women taking HRT compared to those not taking HRT. The number of additional cases of stroke caused by HRT increases with age.
Comparison
In women aged 50-59 who do not take HRT, the estimated number of cases of stroke over a 5-year period is 8 out of 1000 women.
In women aged 50-59 who take HRT, the number of cases over a 5-year period will be 11 out of 1000 women (i.e., 3 additional cases).
Other conditions
HRT does not prevent memory loss. The risk of memory loss may be slightly higher in women who start HRT at an age over 65. For more information, see 2. Important information before taking Kliogest.
Kliogest and other medicines
Certain medicines may affect the efficacy of Kliogest, which may lead to irregular bleeding. These include:
- antiepileptic drugs(such as phenobarbital, phenytoin, and carbamazepine),
- antituberculosis drugs(such as rifampicin, rifabutin),
- medicines used to treat HIV infection(such as nevirapine, efavirenz, ritonavir, and nelfinavir),
- medicines used to treat hepatitis C(such as telaprevir),
- herbal products containing St. John's Wort(Hypericum perforatum).
HRT may affect the action of other medicines:
- the antiepileptic drug lamotrigine, which may increase the frequency of seizures,
- medicines used to treat hepatitis C (e.g., the combination treatment ombitasvir/paritaprevir/ritonavir with dasabuvir or without dasabuvir, as well as the combination glecaprevir/pibrentasvir) may cause increased liver blood test results (increased ALT enzyme activity) in women taking combined hormonal contraceptives containing ethinyl estradiol. Kliogest contains estradiol, not ethinyl estradiol. It is not known whether taking Kliogest with this combination treatment may increase ALT enzyme activity.
Other medicines that may increase the effect of Kliogest:
- medicines containing ketoconazole(an antifungal drug).
Kliogest may affect concomitant treatment with cyclosporin.
Tell your doctor or pharmacistabout all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription, herbal medicines, or other natural products. Your doctor will advise you on this matter.
Laboratory tests
If a blood test is necessary, inform your doctor or laboratory staff that you are taking Kliogest, as it may affect the results of some tests.
Taking Kliogest with food and drink
The tablets can be taken with or without food and drink.
Pregnancy and breastfeeding
Pregnancy:Kliogest is intended for use in postmenopausal women only.
If pregnancy is confirmed, stop taking Kliogest and consult your doctor immediately.
Breastfeeding:do not take Kliogest during breastfeeding.
Driving and using machines
The effect of Kliogest on the ability to drive and use machines is not known.
Important information about some of the ingredients of Kliogest
Kliogest contains lactose monohydrate. If you have been diagnosed with an intolerance to some sugars, consult your doctor before taking Kliogest.
3. How to take Kliogest
Always take this medicine exactly as your doctor has told you. If you are not sure, check with your doctor or pharmacist.
Take one tablet daily, approximately at the same time.
Swallow the tablet with water.
Take one tablet daily, without interruption.After finishing all 28 tablets in the calendar pack, continue treatment by starting a new pack of Kliogest.
Instructions for using the calendar pack can be found at the end of this leaflet, in the section titled "INSTRUCTIONS FOR THE USER".
Take Kliogest as directed. If treatment requires a change from another HRT product that caused menstrual bleeding, start taking Kliogest immediately after the bleeding has stopped.
Your doctor should prescribe the lowest possible dose for the shortest duration necessary to relieve symptoms. If you think the dose of Kliogest is too high or too low, consult your doctor.
Taking more Kliogest than prescribed
If you have taken more Kliogest than prescribed, consult your doctor or pharmacist as soon as possible. Taking more estrogen than prescribed by your doctor may cause breast tenderness, nausea, vomiting, and/or irregular vaginal bleeding (metrorrhagia). Taking more progestogen than prescribed by your doctor may cause depression, fatigue, acne, or excessive hair growth on the body or face (hirsutism).
Missing a dose of Kliogest
If you forget to take a tablet at the usual time, take it within the next 12 hours. If more than 12 hours have passed, take the next dose at the usual time the next day. Do not take a double dose to make up for a missed dose.
In women with an intact uterus, missing a dose may increase the likelihood of bleeding and spotting.
Stopping Kliogest treatment
Before stopping Kliogest treatment, consult your doctor, who will explain the consequences of stopping treatment and discuss other possible forms of therapy.
If you have any further questions about taking Kliogest, consult your doctor or pharmacist.
If surgery is planned
If you are scheduled to undergo surgery, inform your surgeon that you are taking Kliogest. It may be necessary to stop taking Kliogest 4 to 6 weeks before surgery to reduce the risk of blood clots (see Blood clots in the veins (venous thromboembolism)). Before restarting Kliogest, consult your doctor.
4. Possible side effects
Like all medicines, Kliogest can cause side effects, although not everybody gets them.
Women taking HRT are at increased risk of developing the following diseases compared to women not taking HRT:
- breast cancer;
- endometrial hyperplasia or cancer (cancer of the uterine lining);
- ovarian cancer;
- blood clots in the veins of the legs or lungs (venous thromboembolism);
- coronary artery disease;
- stroke;
- possibly memory loss, if HRT is started at an age over 65. For more information, see 2. Important information before taking Kliogest.
Hypersensitivity (allergy) (uncommon side effect - may affect up to 1 in 100 women)
(Uncommon side effect - may affect up to 1 in 100 women)
Although hypersensitivity (allergy) is an uncommon side effect, it can occur. Symptoms of hypersensitivity (allergy) may include one or more of the following:
hives, itching, swelling, difficulty breathing, low blood pressure (pale and cold skin, rapid heartbeat), dizziness, sweating, which may be symptoms of anaphylaxis and/or anaphylactic shock. If any of the above symptoms occur, stop taking Kliogest and seek medical help immediately.
Very common side effects (may affect more than 1 in 10 women)
- breast tenderness or pain,
- vaginal bleeding.
Common side effects (may affect up to 1 in 10 women)
- headache,
- weight gain due to fluid retention,
- vaginal inflammation,
- onset or worsening of migraine,
- fungal vaginal infection,
- onset or worsening of depression,
- nausea,
- abdominal pain, feeling of bloating or discomfort in the abdomen,
- breast enlargement or swelling,
- back pain,
- leg cramps,
- uterine fibroids (fibroids), worsening, occurrence, or recurrence,
- peripheral edema (swelling of hands and feet),
- weight gain.
Uncommon side effects (may affect up to 1 in 100 women)
- bloating or gas,
- acne,
- hair loss (alopecia),
- excessive hair growth (hirsutism),
- itching or hives,
- superficial vein thrombosis,
- ineffectiveness of the medicine,
- allergic reactions,
- nervousness.
Rare side effects (may affect up to 1 in 1000 women)
- blood clots in the blood vessels of the legs or lungs (deep vein thrombosis, pulmonary embolism).
Very rare side effects (may affect up to 1 in 10,000 women)
- endometrial cancer (cancer of the uterine lining),
- endometrial hyperplasia (excessive thickening of the uterine lining),
- high blood pressure, worsening of hypertension,
- gallbladder disease, onset or recurrence of gallstones, worsening of symptoms,
- increased sebum production, onset of acne,
- acute or recurrent swelling (angioedema),
- insomnia, dizziness, anxiety,
- changes in libido,
- vision disturbances,
- weight loss,
- vomiting,
- heartburn (dyspepsia),
- vulvar and vaginal itching,
- heart attack and stroke,
- generalized allergic reactions (anaphylaxis, anaphylactic shock).
Other side effects of combined HRT
The following side effects have been reported during treatment with other HRT medicines:
- various skin disorders:
- skin discoloration, especially on the face or neck, known as "pregnancy patches" (chloasma),
- red, painful lumps (erythema nodosum),
- rash, including erythema multiforme (a rash with target-shaped lesions),
- red or purple discoloration of the skin and/or mucous membranes (purpura),
- "dry eyes",
- change in tear composition.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Ministry of Health:
Jerozolimskie Avenue 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Kliogest
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after "EXP". The expiry date refers to the last day of the month.
Store below 25°C.
Do not store in the refrigerator.
Keep the medicine in its outer packaging to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Kliogest contains
- The active substances of Kliogest are estradiol 2 mg (as estradiol hemihydrate) and norethisterone acetate 1 mg.
- The other ingredients of Kliogest are lactose monohydrate, maize starch, hydroxypropyl cellulose, talc, and magnesium stearate.
- The tablet coating contains hypromellose, triacetin, and talc.
What Kliogest looks like and contents of the pack
The tablets are white, biconvex, and 6 mm in diameter. The tablets have "NOVO 281" embossed on them.
Pack sizes:
- 28 coated tablets.
Marketing authorization holder and manufacturer
Novo Nordisk A/S
Novo Allé
2880 Bagsværd, Denmark.
For more information, contact the local representative of the marketing authorization holder:
Novo Nordisk Pharma Sp. z o.o.
Tel.: 22 444 49 00
Fax: 22 444 49 01
Date of last revision of the leaflet:
Other sources of information
Detailed information about this medicine can be found on the website of the Ministry of Health: www.mz.gov.pl.
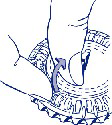
INSTRUCTIONS FOR THE USER
How to use the calendar pack
1. Setting the day indicator
Turn the inner dial so that the day of the week is opposite the small plastic flap.
2. Removing the first tablet
Break off the plastic flap and remove the first tablet.
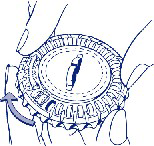
3. Moving the dial each day
The next day, turn the transparent dial clockwise, as indicated by the arrow. Remove the next tablet. Remember to take only one tablet per day.
The transparent part can only be turned after the tablet has been removed from the opening.


- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterNovo Nordisk A/S
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KliogestDosage form: Tablets, 1 mg + 0.5 mgActive substance: norethisterone and estrogenManufacturer: Novo Nordisk A/SPrescription requiredDosage form: Tablets, 1 mg + 2 mgActive substance: norethisterone and estrogenManufacturer: Bayer AGPrescription requiredDosage form: Tablets, 1 mg + 0.5 mgActive substance: norethisterone and estrogenPrescription required
Alternatives to Kliogest in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Kliogest in Испания
Online doctors for Kliogest
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Kliogest – subject to medical assessment and local rules.






