
Kivizidiale

Ask a doctor about a prescription for Kivizidiale

How to use Kivizidiale
Leaflet attached to the packaging: information for the user
Kivizidiale,
40 micrograms/ml + 5 mg/ml, eye drops, solution
Trawoprost + Timolol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Kivizidiale and what is it used for
- 2. Important information before using Kivizidiale
- 3. How to use Kivizidiale
- 4. Possible side effects
- 5. How to store Kivizidiale
- 6. Contents of the packaging and other information
1. What is Kivizidiale and what is it used for
Kivizidiale eye drops, solution is a combination of two active substances (travoprost and timolol). Travoprost is a prostaglandin analogue that works by increasing the outflow of aqueous fluid from the eye, which reduces pressure. Timolol is a beta-blocker that works by reducing the production of fluid inside the eye. Both of these substances work together to reduce the pressure inside the eye.
Kivizidiale in the form of eye drops is used to treat high pressure in the eye in adults, including the elderly. This pressure can lead to the development of a disease called glaucoma.
Kivizidiale eye drops, solution is a sterile solution that does not contain preservatives.
2. Important information before using Kivizidiale
When not to use Kivizidiale eye drops, solution
- if the patient has been diagnosed with an allergy to travoprost, prostaglandins, timolol, beta-blockers, or any of the other ingredients of this medicine (listed in section 6).
- if the patient has or has had respiratory problems such as asthma, severe chronic obstructive pulmonary disease (a serious lung disease that can cause wheezing, difficulty breathing, and/or chronic cough) or other types of respiratory problems.
- if the patient has severe hay fever.
- if the patient has a slow heart rate, heart failure, or heart rhythm disorders (irregular heartbeat).
- if the patient's eye surface is cloudy.
Ask your doctor if any of the above conditions apply to you.
Warnings and precautions
Before starting to use this medicine, tell your doctor about any medical conditions you have or have had, especially:
- coronary heart disease (symptoms may include chest pain or tightness, difficulty breathing, shortness of breath), heart failure, low blood pressure.
- heart rhythm disorders, such as slow heartbeat.
- breathing difficulties, asthma, or chronic obstructive pulmonary disease.
- diseases with impaired blood circulation (such as Raynaud's disease or Raynaud's syndrome).
- diabetes (as timolol may mask the symptoms of low blood sugar).
- hyperthyroidism (as timolol may mask the objective and subjective symptoms of thyroid disease).
- myasthenia gravis (a chronic neuromuscular weakness).
- cataract surgery.
- eye inflammation.
If the patient is to undergo any surgical procedure, they should inform their doctor about the use of Kivizidiale, as timolol may affect the action of certain anesthetics.
If, while using Kivizidiale, the patient experiences any severe allergic reaction (skin rash, redness, and itching of the eye), treatment with adrenaline may not be effective enough. Therefore, it is essential to tell the doctor about the use of Kivizidiale if the patient is to receive any other treatment.
Kivizidiale may change the color of the iris (the colored part of the eye); this change may be permanent.
Kivizidiale may increase the length, thickness, color, and/or number of eyelashes and may cause abnormal hair growth on the eyelids.
Travoprost may be absorbed through the skin, and therefore, should not be used by pregnant or breastfeeding women. If any amount of the medicine gets on the skin, it should be washed off immediately.
Children and adolescents
Kivizidiale is not intended for use in children and adolescents under 18 years of age.
Kivizidiale and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take, including those available without a prescription.
Kivizidiale may affect the action of other medicines taken at the same time or other medicines may affect the action of Kivizidiale; this also applies to other eye drops used to treat glaucoma.
Tell your doctor about taking or planning to take other medicines, including:
- blood pressure-lowering medicines,
- medicines used to treat heart diseases, including quinidine (used to treat heart diseases and some forms of malaria),
- antidiabetic medicines or antidepressants, such as fluoxetine and paroxetine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
Do not use Kivizidiale during pregnancy, unless your doctor advises you to do so. If you can become pregnant, use appropriate contraceptive methods while using this medicine.
Do not use Kivizidiale if you are breastfeeding. Kivizidiale may pass into breast milk.
Driving and using machines
For some time after using Kivizidiale, vision may be blurred. Do not drive or operate machinery until this effect wears off.
Kivizidiale contains macrogolglycerol hydroxystearate 40
This medicine contains macrogolglycerol hydroxystearate 40, which may cause skin reactions.
3. How to use Kivizidiale
Always use this medicine exactly as your doctor has told you. If you are not sure, consult your doctor or pharmacist.
Recommended dose
One drop into the conjunctival sac of the affected eye(s) once daily: in the morning or evening.
Use the medicine every day at the same time.
Kivizidiale can be used in both eyes only if advised by your doctor.
Use Kivizidiale for as long as your doctor recommends.
Use Kivizidiale only as eye drops.
If you are using other eye drops, wait at least 5 minutes between using Kivizidiale and other eye drops.
If you wear soft contact lenses, do not use Kivizidiale while wearing them. After using Kivizidiale, wait 15 minutes before putting your lenses back in.
Instructions for use
1a 1b |
|
2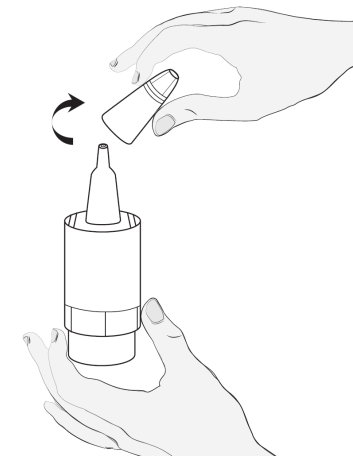 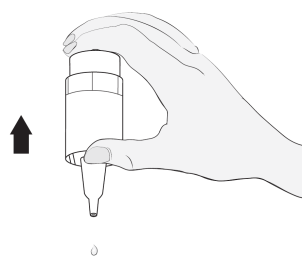 |
|
3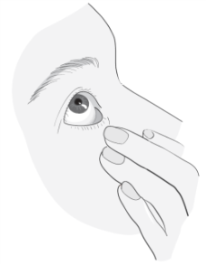 |
|
4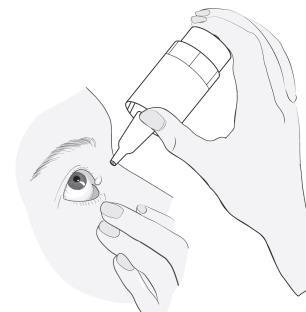 |
|
5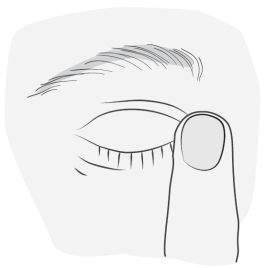 |
|
|
Using more Kivizidiale than recommended
If you use more Kivizidiale than recommended, rinse your eyes with warm water. Do not use more medicine until the next normal dose.
Missing a dose of Kivizidiale
If you miss a dose of Kivizidiale, continue using it as usual. Do not use a double dose to make up for the missed dose. The dose of medicine for the affected eye(s) should not exceed one drop per day.
Stopping the use of Kivizidiale
If you stop using Kivizidiale without consulting your doctor, the pressure in your eye will not be controlled, which can lead to vision loss.
If you have any doubts about using the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Kivizidiale can cause side effects, although not everybody gets them.
Usually, you can continue using the drops, unless the side effects are severe.
If you are concerned, talk to your doctor or pharmacist. Do not stop using Kivizidiale without consulting your doctor.
Very common side effects(may affect more than 1 in 10 people)
Eyelid changes
Common side effects(may affect up to 1 in 10 people)
Eyelid changes
Uncommon side effects(may affect up to 1 in 100 people)
Eyelid changes
Rare side effects(may affect up to 1 in 1,000 people)
Eyelid changes
Side effects with unknown frequency(frequency cannot be estimated from the available data)
Eyelid changes
Additional information:
Kivizidiale is a combination of two active substances, travoprost and timolol. Travoprost and timolol (a beta-blocker), like other eye medicines, are absorbed into the bloodstream. This may cause side effects similar to those seen with beta-blockers taken orally or by injection. However, the frequency of side effects with eye medicines is less than with oral medicines or injections.
The following side effects have been observed with the entire group of beta-blockers used in ophthalmology or with travoprost alone.
Eyelid changes:
Warnings and precautions
- Ear and labyrinth disorders:dizziness with a feeling of spinning, ringing in the ears.
- Heart and blood vessel disorders:slow heart rate, irregular heartbeat, swelling (fluid retention), changes in heart rate or rhythm, heart failure, heart attack, low blood pressure, Raynaud's syndrome, cold hands and feet, decreased blood flow to the brain.
- Respiratory disorders:bronchospasm in the lungs (mainly in patients with pre-existing disease), runny nose or feeling of nasal congestion, sneezing (caused by allergy), difficulty breathing, nosebleeds, dryness of the nasal mucosa.
- Nervous system and general disorders:difficulty sleeping (insomnia), nightmares, memory loss, loss of strength and energy, anxiety (excessive emotional distress).
- Gastrointestinal disorders:taste disturbances, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting, and constipation.
- Allergic reactions:exacerbation of allergic symptoms, generalized allergic reactions including skin swelling on areas such as the face and limbs, which can block the airways and cause difficulty swallowing and breathing, or localized and generalized rash, itching, and severe life-threatening allergic reactions.
- Skin and subcutaneous tissue disorders:psoriasis-like skin rash or worsening of psoriasis, skin peeling, abnormal hair structure, skin inflammation with itching rash and redness, hair color change, eyelash loss, itching, abnormal hair growth, skin redness.
- Musculoskeletal and connective tissue disorders:worsening of myasthenia gravis symptoms (a chronic neuromuscular weakness), abnormal sensations such as tingling and numbness, muscle weakness/fatigue, muscle pain not caused by exercise, joint pain.
- Renal and urinary disorders:difficult and painful urination, urinary incontinence.
- Reproductive system and breast disorders:sexual dysfunction, decreased libido.
- Metabolic and nutritional disorders:low blood sugar, increased prostate cancer marker values.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Tel.: +48 22 49 21 301
Fax: +48 22 49 21 309
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Kivizidiale
Keep out of the sight and reach of children.
Do not use Kivizidiale eye drops, solution after the expiry date (marked as "EXP") on the container and carton. The expiry date refers to the last day of that month.
Do not use this medicine if the container is visibly damaged or broken before the first opening.
Store in a temperature below 25°C.
Discard the container 28 days after first openingand start using a new container. Write the opening date of each container on the space provided on the label of the container and on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Kivizidiale contains
The active substances of the medicine are travoprost and timolol.
Each ml of solution contains 40 micrograms of travoprost and 5 mg of timolol (as timolol maleate).
The other ingredients are macrogolglycerol hydroxystearate 40, sodium chloride, propylene glycol, boric acid, mannitol, sodium hydroxide (to adjust pH), purified water.
What Kivizidiale looks like and contents of the pack
Kivizidiale eye drops, solution is a clear, colorless aqueous solution, with a volume of 2.5 ml, practically free from particles.
The carton contains multidose containers made of PP with a capacity of 5 ml, with a pump (made of PP, HDPE, LDPE), a pressure cylinder, and a cap made of HDPE.
Package sizes: 1 or 3 containers containing 2.5 ml of solution, in a carton.
Not all package sizes may be marketed.
Marketing authorization holder
Bausch + Lomb Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer
JADRAN - GALENSKI LABORATORIJ d.d.
Svilno 20
51000 Rijeka
Croatia
Pharmathen SA
Dervenakion 6
15351 Pallini Attiki
Greece
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Kivizidiale 40 Mikrogramm/ml + 5 mg/ml Augentropfen Lösung
Belgium
Kivizidiale 40 microgram/ml + 5 mg/ml oogdruppels, oplossing
Bulgaria
Кивизидиал 40 микрограма/ml + 5 mg/ml капки за очи, разтвор
Germany
Kivizidiale 40 Microgramm/ml + 5 mg/ml Augentropfen, Lösung
Cyprus
Kivizidiale
Croatia
Kivizidiale 40 mikrograma/ml + 5 mg/ml, kapi za oko, otopina
Denmark
Kivizidiale
Estonia
Kivizidiale
Spain
Kivizidiale 40 μg/ml + 5 mg/ml colirio en solución
France
Kivizidiale, 40 microgrammes / 5 mg par mL collyre en solution
Greece
Kivizidiale
Hungary
Kivizidiale 40 mikrogramm/ml + 5 mg/ml oldatos szemcsepp
Netherlands
Kivizidiale 40 microgram/ml + 5 mg/ml oogdruppels, oplossing
Lithuania
Kivizidiale 40 mikrogramų / 5 mg/ ml akių lašai (tirpalas)
Luxembourg
Kivizidiale 40 microgrammes/ml + 5 mg/ml collyre en solution
Poland
Kivizidiale
Portugal
Kivizidiale 40 μg/ml + 5 mg/ml colírio, solução
Romania
Kivizidiale 40 micrograme/mL + 5 mg/mL picături oftalmice, soluţie
Slovakia
Kivizidiale 40 mikrogramov/ml + 5 mg/ml
Date of last revision of the leaflet: March 2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d. Pharmaten S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KivizidialeDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Kivizidiale in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Kivizidiale in Spain
Alternative to Kivizidiale in Ukraine
Online doctors for Kivizidiale
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Kivizidiale – subject to medical assessment and local rules.














