How to use Implanon Nxt
Leaflet accompanying the packaging: information for the user
Implanon NXT, 68 mg, subcutaneous implant
Etonogestrel
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor, pharmacist, or nurse.
- If the patient experiences any adverse reactions, including any adverse reactions not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
The patient will receive a Patient Warning Card from their doctor, which contains important information that they should know. The card should be kept in a safe place and shown during each visit related to the use of the subcutaneous implant.
Table of contents of the leaflet
- 1. What Implanon NXT is and what it is used for
- 2. Important information before using Implanon NXT
- 3. How to use Implanon NXT
- 4. Possible side effects
- 5. How to store Implanon NXT
- 6. Contents of the packaging and other information
- 7. Information intended only for healthcare professionals
1. What Implanon NXT is and what it is used for
Implanon NXT is a contraceptive subcutaneous implant placed in a single-use applicator. The safety and efficacy of the medicinal product have been established in women aged
- 18-40 years. The subcutaneous implant is a small, soft, flexible, plastic rod 4 cm long and 2 mm in diameter, which contains 68 milligrams of the active substance - etonogestrel. The applicator allows the doctor to place the subcutaneous implant directly under the skin of the arm. Etonogestrel is a synthetic female hormone similar to progesterone. A small amount of etonogestrel is constantly released into the bloodstream. The subcutaneous implant itself is made of ethylene and vinyl acetate copolymer, a plastic that does not dissolve in the body. The subcutaneous implant also contains a small amount of barium sulfate, which makes it visible during X-ray examination.
Implanon NXT is used to prevent pregnancy.
How Implanon NXT works
The subcutaneous implant is inserted directly under the skin. The active substance, etonogestrel, works in two ways:
- It prevents the release of an egg from the ovary.
- It causes changes in the cervical mucus, making it difficult for sperm to enter the uterus.
As a result of its action, Implanon NXT prevents pregnancy for a period of three years, although if the patient is overweight, the doctor may recommend earlier replacement of the subcutaneous implant. Implanon NXT is one of several methods of preventing pregnancy. Another commonly used contraceptive method is the combined oral contraceptive pill. Unlike the combined oral contraceptive pill, Implanon NXT can be used by women who cannot or do not want to use estrogens. When using Implanon NXT, there is no need to remember to take a daily pill. This is one of the reasons why Implanon NXT is considered a very reliable method (over 99% effective). In rare cases, it has been observed that the subcutaneous implant was not inserted correctly or was not inserted at all. This can result in an unplanned pregnancy. During the use of Implanon NXT, menstrual bleeding may change or not occur at all, may occur irregularly, less frequently, more frequently, be prolonged, or rarely be very heavy. Based on the course of bleeding during the first three months, the patient can determine the course of subsequent bleeding. Painful menstruation may be alleviated. It is possible to stop using Implanon NXT at any time (see also: "Stopping the use of Implanon NXT").
2. Important information before using Implanon NXT
Implanon NXT, like other hormonal contraceptives, does not protect against HIV infection (AIDS) or other sexually transmitted diseases.
When not to use Implanon NXT
Implanon NXT should not be used if any of the following conditions are present. The doctor should be informed about their occurrence before inserting Implanon NXT. The doctor may advise a non-hormonal contraceptive method.
- If the patient is allergic to etonogestrel or any of the other ingredients of this medicine (listed in section 6).
- If the patient currently has venous thromboembolic disease. Venous thromboembolic disease is a condition in which blood clots form in the veins [e.g., in the legs (deep vein thrombosis) or lungs (pulmonary embolism)].
- If the patient currently has or has had jaundice (yellowing of the skin), severe liver disease (when the liver does not function properly), or liver tumors.
- If the patient has had or may have breast cancer or genital organ tumors.
- If the patient has bleeding from the genital tract of unknown cause.
If any of the above conditions occur for the first time during the use of Implanon NXT, the doctor should be contacted immediately.
Warnings and precautions
Before starting to use Implanon NXT, the doctor, pharmacist, or nurse should be consulted. If Implanon NXT is used in the presence of any of the diseases listed below, close medical supervision is required. The attending doctor will explain the course of action. Before inserting Implanon NXT, the doctor should be informed about their occurrence. The doctor should also be contacted if any of the following diseases occur or worsen during the use of Implanon NXT:
- History of breast cancer;
- Current or history of liver disease;
- Venous thromboembolic disease;
- Diabetes;
- Overweight;
- Epilepsy;
- Tuberculosis;
- Hypertension;
- Current or history of chloasma (yellow-brown pigmented spots on the skin, especially the face); in these cases, sun exposure or ultraviolet radiation should be avoided.
Possible serious diseases Cancer
The following information is based on clinical trials in women using daily combined oral contraceptives containing two different female hormones ("oral contraceptive pill"). It is not known whether these observations also apply to women using other hormonal contraceptives, such as implants containing only progestogen. Breast cancer was diagnosed slightly more often in women using oral contraceptive pills, but it is not known whether this was caused by their use. For example, it is possible that tumors are detected more often in women using combined oral contraceptive pills because they are examined by a doctor more often. The increased frequency of breast cancer decreases gradually after stopping the use of combined oral contraceptive pills. It is very importantfor the patient to regularly examine her breasts. If she finds a lump, she should contact her doctor.The patient should inform her doctor about the occurrence of breast cancer in her close relatives. In rare cases, benign and very rarely malignant liver tumors have been found in patients using combined oral contraceptive pills. If the patient experiences severe abdominal pain, she should contact her doctor immediately.ThrombosisIn venous thrombosis, blood clots form in the veins. They can occur, for example, in the veins of the lower limbs, lungs (pulmonary embolism), or other organs and parts of the body. A blood clot can also block an artery (so-called arterial thrombosis), which can lead to a heart attack or stroke. The use of combined hormonal contraceptives increases the risk of such blood clots compared to women who do not take any combined hormonal contraceptives. This risk is not as high as the risk of blood clots in pregnant women. It is believed that the risk in women using only progestogens, such as Implanon NXT, is lower than in women using combined oral contraceptive pills containing estrogens .In women using etonogestrel implants, cases of blood clots have been reported, such as pulmonary embolism, deep vein thrombosis, heart attack, and stroke, but available data do not indicate an increased risk of these events in women using the subcutaneous implant.
In case of symptoms of venous thromboembolic disease, such as
the patient should contact their doctor immediately(see also "When to contact a doctor").
Other conditions Changes in menstrual bleeding pattern
During the use of Implanon NXT, as with other progestogen-only contraceptives, menstrual bleeding may be irregular. The frequency (absence of bleeding, rare bleeding, more frequent or prolonged bleeding) and intensity (increased or decreased) of menstrual bleeding may change. Absence of menstruation was observed in 1 in 5 women, while in another 1 in 5 women, more frequent and/or prolonged menstrual bleeding was observed. Very heavy bleeding has been observed occasionally. In clinical trials, irregular menstrual bleeding was the most common reason for discontinuing the subcutaneous implant (about 11%). Based on the course of bleeding during the first three months, women can determine the course of subsequent bleeding. Changes in bleeding patterns do not mean that Implanon NXT is not suitable for the patient and does not show contraceptive efficacy. Generally, no special actions are required. However, if bleeding is very heavy or prolonged, the doctor should be contacted. Events related to the insertion and removal of the implantAs a result of improper insertion of the implant or external force (e.g., manipulation of the implant or contact sports), the subcutaneous implant may move in the arm relative to the insertion site. In rare cases, subcutaneous implants have been found in blood vessels of the arm or in the pulmonary artery (a blood vessel in the lungs). In cases where the subcutaneous implant has moved relative to the insertion site, locating the implant may be more difficult, and removal may require a larger incision or surgical procedure performed in a hospital. If the subcutaneous implant cannot be located in the arm, healthcare professionals may perform a chest X-ray or other imaging test. If the implant is located in the chest, surgery may be necessary. If the subcutaneous implant cannot be located and there is no evidence that it has been expelled, the contraceptive effect and the risk of progestogen side effects may last longer than the woman desires. If the subcutaneous implant is not palpable at any time, the doctor should be contacted as soon as possible. Psychiatric disordersSome women using hormonal contraceptives, including Implanon NXT, have reported depression or low mood. Depression can be severe and sometimes lead to suicidal thoughts. If mood changes and symptoms of depression occur, the doctor should be contacted as soon as possible for further medical advice. Ovarian cystsSmall fluid-filled blisters can develop in the ovaries during the use of contraceptive methods containing low doses of hormones. These are ovarian cysts. They usually disappear on their own. Occasionally, they can cause mild abdominal pain. Only in rare cases can they pose a more serious problem. Fracture or bending of the implantFracture or bending of the subcutaneous implant in the patient's arm should not affect its function. Fracture or bending of the subcutaneous implant may be caused by external forces. A fractured subcutaneous implant may move relative to the insertion site. If there are any questions, the doctor should be contacted.
Implanon NXT and other medicines
The doctor should always be informed about medicines or herbal products currently used by the patient. The doctor of another specialty or the dentist prescribing other medicines (or pharmacist) should also be informed about the use of Implanon NXT. They may inform about the need to use an additional contraceptive method (e.g., a condom), and if so, for how long, as well as whether it is necessary to modify the use of another medicine. Some medicines
- may affect the concentration of Implanon NXT in the blood
- may reduce its contraceptive efficacy
- may cause unexpected bleeding.
This applies to medicines used to treat:
- epilepsy (e.g., primidone, phenytoin, barbiturates, carbamazepine, oxcarbazepine, topiramate, felbamate),
- tuberculosis (e.g., rifampicin),
- HIV infection (e.g., ritonavir, nelfinavir, nevirapine, efavirenz),
- hepatitis C virus infection (e.g., boceprevir, telaprevir),
- other infectious diseases (e.g., griseofulvin),
- high blood pressure in the lungs (bosentan),
- depressive moods (herbal medicine - St. John's Wort (Hypericum perforatum)).
Implanon NXT may affect the action of other medicines, such as:
- cyclosporine
- the antiepileptic drug lamotrigine (which may lead to an increased frequency of seizures).
Before taking any medicine, the doctor or pharmacist should be consulted.
Implanon NXT with food and drink
No effect of food and drink on the action of Implanon NXT has been demonstrated.
Pregnancy and breastfeeding
Implanon NXT should not be used in pregnant women or in suspected pregnancy. If there is any doubt as to whether the woman is pregnant, a pregnancy test should be performed before starting to use Implanon NXT. Implanon NXT can be used during breastfeeding. Although a small amount of the active substance of Implanon NXT passes into breast milk, it does not affect the secretion and quality of breast milk, as well as the growth and development of children. During breastfeeding, before using this medicine, the doctor should be consulted.
Children and adolescents
The safety and efficacy of Implanon NXT have not been studied in adolescents under the age of 18.
Driving and operating machinery
No effect of Implanon NXT on alertness and concentration has been demonstrated.
When to contact a doctor Regular check-ups
Before inserting Implanon NXT, the doctor will take a medical history and measure the patient's blood pressure, and if necessary, order additional tests. If Implanon NXT is inserted, the patient may be asked by the doctor to undergo routine medical examinations after a certain period from the insertion. The frequency and purpose of these visits will depend on the individual needs of the patient. Healthcare professionals should palpate the implant during each follow-up visit.
The doctor should be contacted immediately if:
- there are any changes in health, especially those listed in this leaflet (see also "When not to use Implanon NXT" and "Warnings and precautions"; do not forget the information related to the health of close relatives);
- there are possible signs of venous thromboembolic disease, such as severe pain or swelling of one leg, unexplained chest pain, difficulty breathing, unusual cough, especially with bloody sputum;
- there is sudden, severe abdominal pain or jaundice;
- a breast lump is felt (see also "Cancer");
- there is sudden or severe pain in the lower abdomen;
- there is irregular, heavy vaginal bleeding;
- the patient is to be immobilized (e.g., stay in bed for a long time) or is to undergo surgery (the doctor should be consulted at least 4 weeks in advance);
- pregnancy is suspected;
- the implant is not palpable after insertion or at any time.
3. How to use Implanon NXT
Before inserting Implanon NXT, the doctor should be informed if the patient is pregnant or suspects that she may be pregnant (e.g., during the current menstrual cycle, she had sexual intercourse without using contraceptive methods).
How to use
Implanon NXT should be inserted and removed only by a doctor familiar with the procedure, as described below in the leaflet. The doctor will decide on the most appropriate time for insertion, depending on the individual situation of the patient (e.g., the currently used contraceptive method). If the patient is switching from a hormonal contraceptive method to a subcutaneous implant, it should be inserted between the 1st and 5th day of the natural menstrual cycle to ensure that the patient is not pregnant. If the implant is inserted after the fifth day of the menstrual cycle, the patient should use an additional contraceptive method (e.g., a condom) for the first 7 days after insertion. Before inserting and removing Implanon NXT, the attending doctor will administer local anesthesia. Implanon NXT is inserted directly under the skin on the inner side of the arm of the non-dominant hand (the hand that is not used for writing). A description of the technique for inserting and removing Implanon NXT can be found in section 6.
The implant must be palpable after insertion
After the procedure, it is recommended that the patient palpate the implant to check its presence. A properly inserted implant should be palpable by both the healthcare professional and the patient, who should feel both ends of the implant between the thumb and index finger. It should be noted that palpation does not provide 100% certainty that Implanon NXT has been inserted. If the implant is not palpable immediately after insertion or at any time, the doctor should be contacted as soon as possible. In case of any doubts, a barrier method of contraception (e.g., a condom) should be used until the presence of the implant is confirmed. Once the implant has been located, it should be removed.
Implanon NXT should be removed or replaced with a new one no later than three years after insertion.
Patient Warning Card
To help the patient remember when and where Implanon NXT was inserted and when it should be removed at the latest, the doctor will provide a Patient Warning Card with this information. The Patient Warning Card also contains instructions for the patient to palpate the implant from time to time to ensure that she feels its location. The patient should contact the doctor as soon as possible if she cannot feel the implant at any time. This card should be kept in a safe place! The Patient Warning Card should be shown to healthcare professionals during each visit related to the use of Implanon NXT. If the patient wants to replace Implanon NXT, a new implant can be inserted immediately after the removal of the previous one. The new implant can be inserted in the same arm and in the same location as the previous one, provided that the previous insertion site was correct. The attending doctor will provide advice.
Stopping the use of Implanon NXT
It is possible to stop using Implanon NXT at any time. If the implant is not palpable, healthcare professionals may determine the location of the implant using X-ray, ultrasound, or magnetic resonance imaging. Depending on the exact location of the implant, removal may be difficult and may require surgical intervention. If the patient does not want to become pregnant, after the removal of the implant, she should ask her doctor about another contraceptive method. If the patient is planning a pregnancy, it is usually recommended to wait until the first natural menstrual period, which will make it easier to determine the expected date of delivery.
4. Possible side effects
Like all medicines, Implanon NXT can cause side effects, although not everybody gets them. During the use of Implanon NXT, menstrual bleeding may occur irregularly. There may be slight spotting, not requiring the use of pads, or more heavy bleeding, which looks more like a scanty menstrual period, when pads are needed. Menstrual bleeding may also not occur at all. Irregular bleeding is not a sign of decreased contraceptive efficacy of Implanon NXT. It does not require any special actions. However, if bleeding is very heavy or prolonged, the doctor should be contacted. Serious side effects, such as cancer and thrombosis, are described in section 2. The patient should read this section for additional information and contact the doctor immediately if necessary. The following side effects have been reported:
| Very common (may occur in more than 1 in 10 women) | Common (may occur in less than 1 in 10 women) | Uncommon (may occur in less than 1 in 100 women) |
| Acne; headaches; weight gain; breast tenderness and pain; irregular menstrual bleeding; vaginal infections. | Hair loss; dizziness; depressive moods; emotional instability; nervousness; decreased libido; increased appetite; abdominal pain; nausea; bloating; painful menstrual bleeding; weight loss; flu-like symptoms; pain; fatigue; hot flashes; pain at the implant insertion site; local reactions at the implant insertion site; ovarian cysts. | Itching; itching in the genital area; rash; excessive hair growth; migraine; anxiety; insomnia; drowsiness; diarrhea; vomiting; constipation; urinary tract infections; discomfort in the vagina (e.g., vaginal discharge); breast enlargement; breast discharge; back pain; fever; fluid retention; painful or difficult urination; allergic reactions; throat inflammation and pain; cold; joint pain; muscle pain; musculoskeletal pain. |
In addition to the side effects listed above, increased blood pressure has been reported. Increased intracranial pressure (mild intracranial hypertension) with symptoms such as persistent headache, as well as nausea, vomiting, and changes in vision, including blurred vision, have been observed. Skin fattening has also been observed. Immediate medical attention should be sought if severe allergic reactions occur, such as facial, tongue, or throat swelling; difficulty swallowing; or hives and difficulty breathing. During the insertion and removal of Implanon NXT, bruising (in some cases severe), pain, swelling, or itching may occur, and in rare cases, infection may occur. A scar or abscess may form at the insertion site. After insertion, the patient may feel weakness. A feeling of numbness or tingling (or loss of sensation) may occur. It is possible for the implant to be expelled or to move, especially if it was not inserted correctly. Rarely, subcutaneous implants have been found in blood vessels, including the pulmonary artery, which may be associated with symptoms such as shortness of breath and (or) cough with or without bleeding. During the removal of the implant, surgical intervention may be necessary. In women using etonogestrel implants, cases of blood clots have been reported in the veins (so-called venous thrombosis) or arteries (so-called arterial thrombosis). A blood clot can block the veins in the legs (deep vein thrombosis), lungs (pulmonary embolism), or other organs. A blood clot can also block an artery, which can lead to a heart attack or stroke. If any side effects occur, including any side effects not listed in the leaflet, the doctor, pharmacist, or nurse should be consulted.
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, the doctor, pharmacist, or nurse should be informed. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Implanon NXT
The medicine should be stored out of sight and reach of children. This medicine should not be used after the expiry date stated on the blister and carton after EXP. Store in the original packaging. Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer required. This will help protect the environment. There are no special precautions for storage.
6. Contents of the packaging and other information
What Implanon NXT contains
Each applicator contains one subcutaneous implant with
- The active substance of the medicine is etonogestrel (68 mg).
- Other ingredients are: Core: Ethylene and vinyl acetate copolymer (28% vinyl acetate) Barium sulfate Magnesium stearate Surface layer: Ethylene and vinyl acetate copolymer (15% vinyl acetate)
What Implanon NXT looks like and what the pack contains
Implanon NXT is a long-acting subcutaneous contraceptive. The single-use, innovative, easy-to-use applicator contains a non-radiopaque implant containing only progestogen. The implant is 4 cm long and 2 mm in diameter, white in color, and contains etonogestrel and barium sulfate. The applicator is specially designed to allow the doctor to insert the subcutaneous implant directly under the skin on the inner side of the arm (non-dominant hand). The subcutaneous implant should be inserted and removed only by a doctor familiar with the procedure. To ensure that the implant can be removed without problems, it should be inserted directly under the skin (see below in the leaflet). During the insertion and removal of the implant, local anesthesia should be used. The risk of complications is low if the following instructions are followed. The packaging sizes are: a cardboard box containing 1 blister, a cardboard box containing 5 blisters. Not all packaging sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Organon Polska Sp. z o.o. ul. Marszałkowska 126/134 00-008 Warsaw Tel.: +48 22 105 50 01 [email protected]
Manufacturer:
N.V. Organon, Kloosterstraat 6, 5349 AB Oss, Netherlands
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Belgium, Germany, Ireland, Luxembourg, Malta, Netherlands, Slovakia, Poland, Portugal, Spain: Implanon NXT Denmark, Estonia, Finland, France, Iceland, Italy, Norway, Romania, Sweden, United Kingdom (Northern Ireland): Nexplanon
Date of last revision of the leaflet: 05/2024
Note:
The following pictograms are intended for the patient who has had the implant inserted and are intended to illustrate the procedure for inserting and removing the implant.
Note: The detailed procedure for inserting and removing Implanon NXT by healthcare professionals is described in the Summary of Product Characteristics and in this patient leaflet in section 7.
6.1. How to insert Implanon NXT
- The insertion of Implanon NXT should only be performed by medical personnel familiar with the procedure.
- To facilitate the insertion of the implant, the patient should lie on her back with her arm bent at the elbow and her hand under her head (or in a position as close to this as possible).
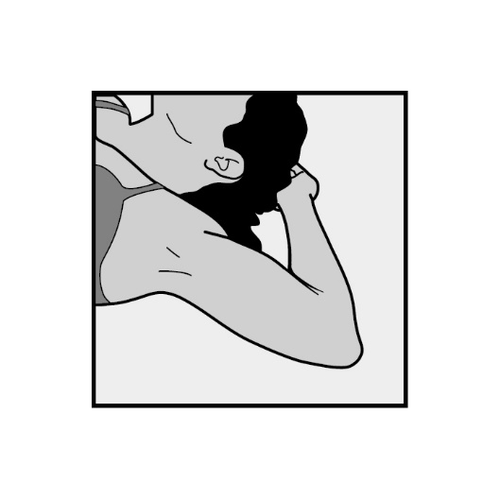
- The implant will be inserted on the inner side of the arm of the non-dominant hand (the hand that is not used for writing).
- The insertion site will be marked on the skin and then disinfected and anesthetized.
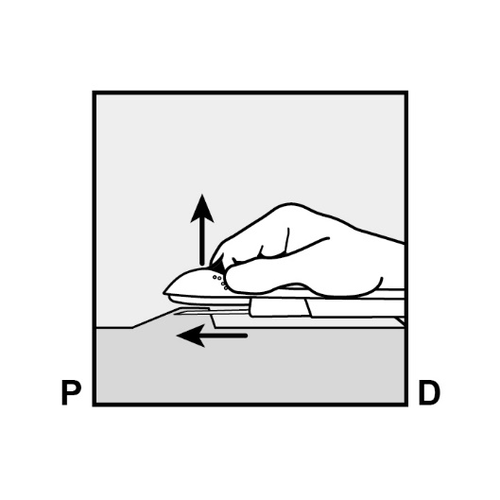
- The skin will be stretched, and the needle will be inserted directlyunder the skin. When the tip of the needle is under the skin, the entire needle will be inserted parallel to the skin surface.
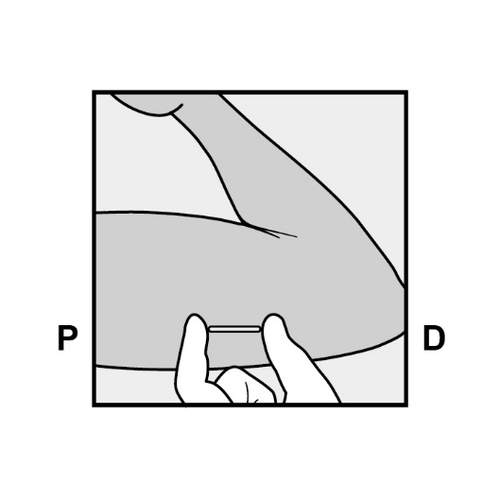
P, proximally (toward the shoulder); D, distally (toward the elbow)
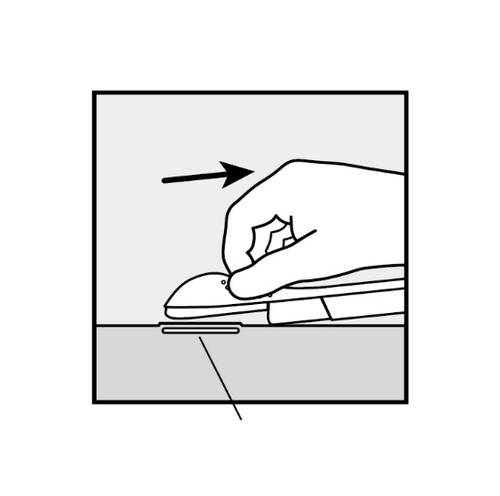
- The doctor will release the purple slider to withdraw the needle from the arm. The implant will remain in the upper arm after the needle is withdrawn.
P D
- It is recommended to check if the implant is palpable (by touch) immediately after insertion. A properly inserted implant should be easily palpable between the thumb and index finger by both the healthcare professional and the patient. It should be noted that palpation does not provide 100% certainty that Implanon NXT has been inserted.
Implanon NXT
- In case of doubt about the presence of the implant, other methods should be used to confirm its presence.
- After locating the implant that could not be palpated, it should be removed.
- Until the presence of the implant is finally confirmed, a barrier method of contraception (e.g., a condom) should be used.
- The insertion site will be covered with a small bandage and a pressure bandage to minimize the risk of bruising. The pressure bandage can be removed after 24 hours, and the small bandage after 3-5 days from insertion.
- After the insertion of the implant, the patient will receive a Patient Warning Card from the healthcare professional with information about the insertion site, date of insertion, and date of removal or replacement. This card should be kept in a safe place, as the information it contains will facilitate the removal of the implant in the future.
6.2. How to remove Implanon NXT
- The implant should be removed by healthcare professionals or medical personnel familiar with the procedure.
- The implant is removed at the patient's request or no later than three years after insertion.
- Detailed information about the location of the implant is contained in the Patient Warning Card.
- Healthcare professionals will determine the location of the implant. If it cannot be located, healthcare professionals may perform an X-ray, computed tomography, ultrasound, or magnetic resonance imaging.
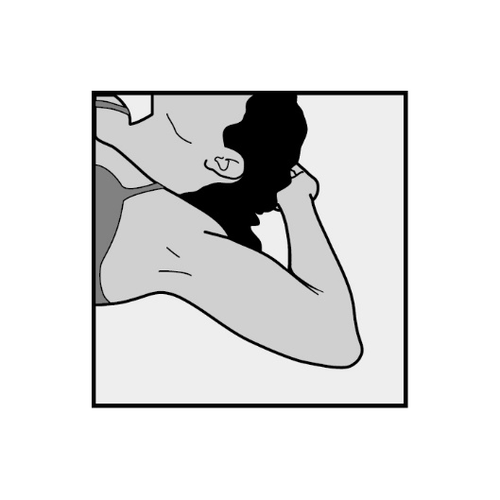
- To facilitate the removal of the implant, the patient should lie on her back with her arm bent at the elbow and her hand under her head (or in a position as close to this as possible).
- The upper arm will be disinfected and anesthetized.
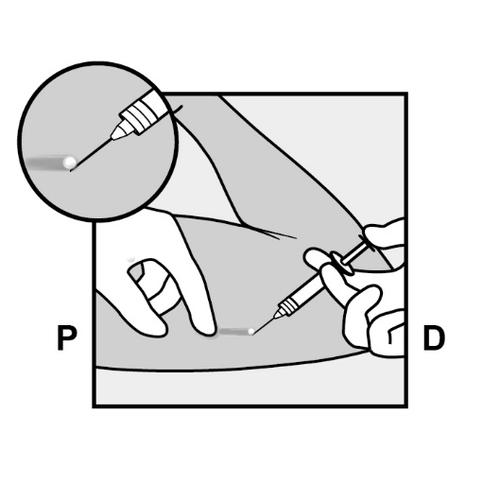
- A small incision will be made along the arm, directly below the end of the implant.
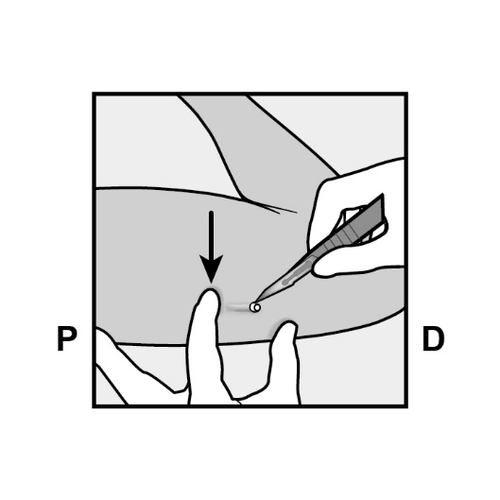
- The implant will be gently pushed toward the incision and removed using forceps.
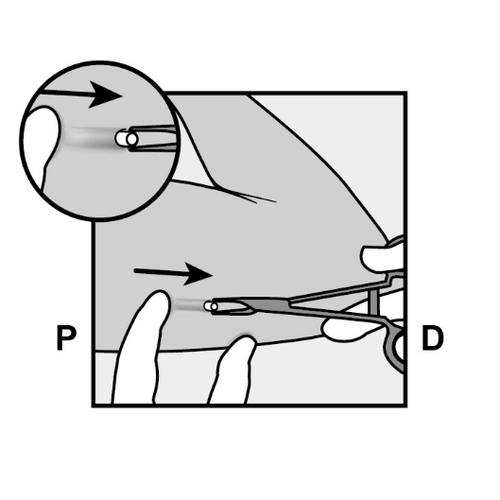
- Occasionally, the implant may be surrounded by dense tissue. In this case, it is necessary to incise the tissue before removing the implant.
- If the patient wants the healthcare professional to replace Implanon NXT with another implant, the new implant can be inserted through the same incision, provided that the previous insertion site was correct.
- The incision will be closed with sterile wound closure strips.
- A pressure bandage will be applied to minimize the risk of bruising. The patient can remove the pressure bandage after 24 hours, and the sterile wound closure strips after 3-5 days.
Information intended only for healthcare professionals:
7. Information intended only for healthcare professionals
7.1 When to insert Implanon NXT
IMPORTANT: Before inserting the implant, it should be ensured that the patient is not pregnant.
The timing of the insertion of the implant depends on the previously used contraceptive method:
If no hormonal contraceptive was used in the previous month
The implant should be inserted between the 1st and 5th day of the natural menstrual cycle, even if the woman is still bleeding (1st day of the cycle is the 1st day of menstrual bleeding). If the implant is inserted as recommended, there is no need to use additional contraceptive methods. If the timing of the insertion of the implant deviates from the recommended, the woman should be advised to use a barrier method for 7 days after the insertion. If sexual intercourse took place, it should be ensured that the patient is not pregnant.
7.2 How to Insert Implanon NXT
To ensure effective action and uncomplicated removal of the Implanon NXT implant, it is essential to insert it correctly and carefully under the skin of the non-dominant arm, following the instructions.
Both the healthcare provider and the patient should be able to palpate the implant after its insertion.
The implant should be inserted under the skin on the inner side of the upper arm of the non-dominant arm.
- The implant inserted deeper than subcutaneously (deep insertion) may be non-palpable, which can make its localization and/or removal difficult (see section 4.2 and 4.4 of the Summary of Product Characteristics).
- If the implant is inserted too deeply, nerves or blood vessels may be damaged.
- Deep or incorrect insertion of the implant may lead to paresthesia (due to nerve damage) and implant displacement (due to intramuscular or fascial placement), and in rare cases, may result in intravascular injection.
Insertion of the Implanon NXT implant should be performed under aseptic conditions and by a trained healthcare provider familiar with the procedure.
The Implanon NXT implant should only be inserted using the specially designed applicator.
Inserting the Implant
To ensure that the implant is inserted just under the skin, the healthcare provider should position themselves to see the needle being inserted, observing the applicator from the side, not from above the arm.
The insertion site and the movement of the needle just under the skin will be clearly visible from the side.
- The patient should be placed on her back with her non-dominant arm bent at the elbow, with her hand under her head (or in a similar position) (Figure 1).
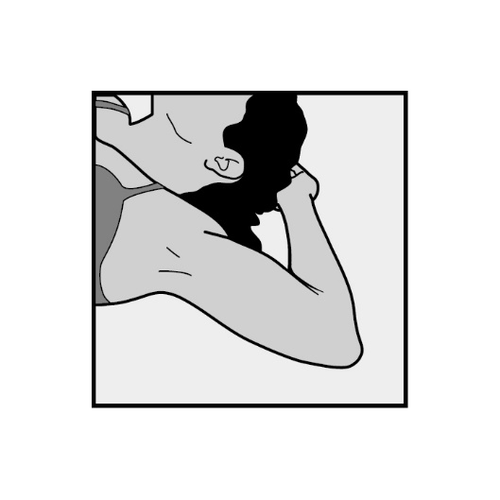
Figure 1
- Identify the insertion site on the inner side of the upper arm of the non-dominant arm.
The insertion site is located above the triceps muscle, about 8-10 cm from the medial epicondyle of the humerus and 3-5 cm posteriorly (below) from the groove between the biceps and triceps muscles (Figure 2a, 2b, and 2c).
This location avoids major blood vessels and nerves in the groove.
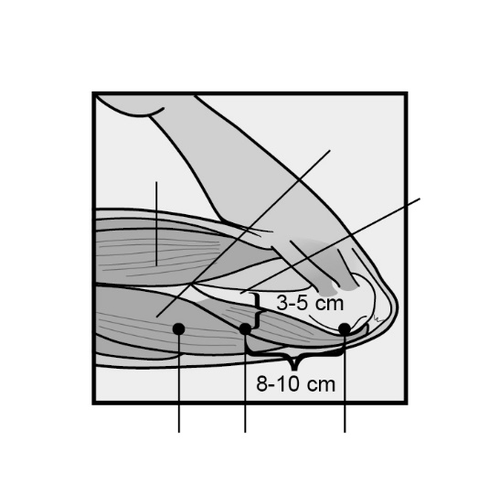
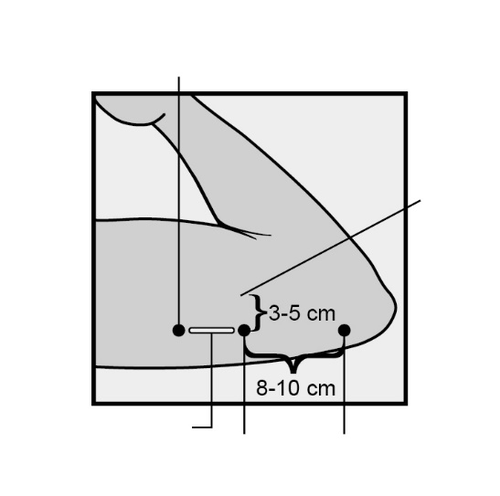
Figure 2a
Figure 2b
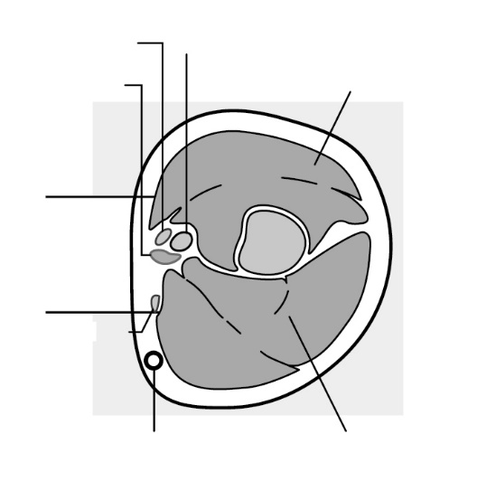
Figure 2c
Cross-section of the upper arm, view from the elbow side
Inner side of the arm
Outer side of the arm
- After marking the arm, confirm the correct position of the insertion site on the inner side of the arm.
- Clean the skin with an antiseptic solution from the insertion site to the marking.
- Administer local anesthesia (e.g., using a spray or injecting 2 ml of 1% lidocaine solution just under the skin, along the "implant insertion channel").
- Remove the Implanon NXT implant from its blister packaging.
Before use, visually inspect the packaging for any damage (e.g., tears, punctures).
If the packaging is damaged, which could affect sterility, do not use the applicator. - Hold the applicator just above the needle, where the surface is textured.
Remove the needle cover by sliding it horizontally off the needle in the direction indicated by the arrow (Figure 3).
If the needle cover cannot be easily removed, do not use the applicator.
Visually verify the presence of the implant, which should be visible as a white rod at the tip of the needle.
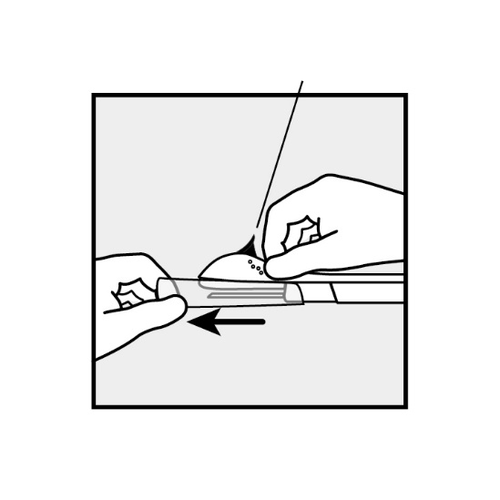
Figure 3
- If the purple slider is released prematurely, start the procedure again with a new applicator.
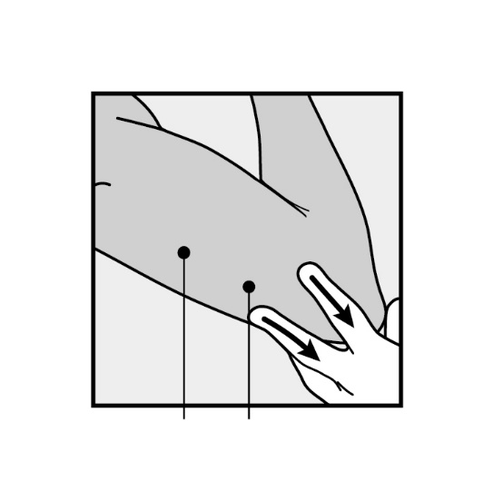
Figure 4
- The implant should be inserted just under the skin(see section 4.4 of the Summary of Product Characteristics).
To ensure that the implant is inserted just under the skin, position yourself to see the needle being inserted, observing the applicator from the side, not from above the arm.
The insertion site and the movement of the needle just under the skin can be seen clearly from the side (see Figure 6).
- Insert the needle at a slight angle (less than 30°) (Figure 5a).
- Insert just the tip of the needle (the beveled opening at the tip) just under the skin, but no further (Figure 5b).
If the needle is inserted too deeply, withdraw it so that only the tip remains under the skin.
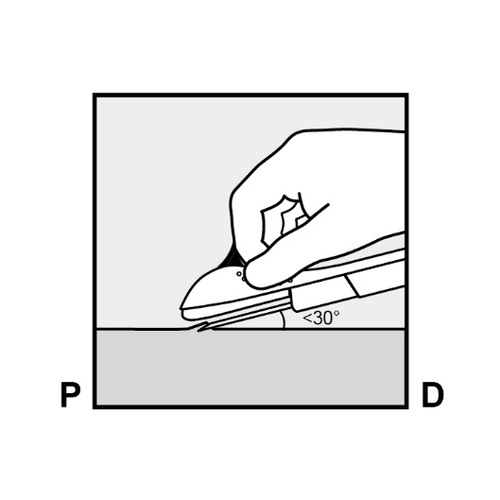
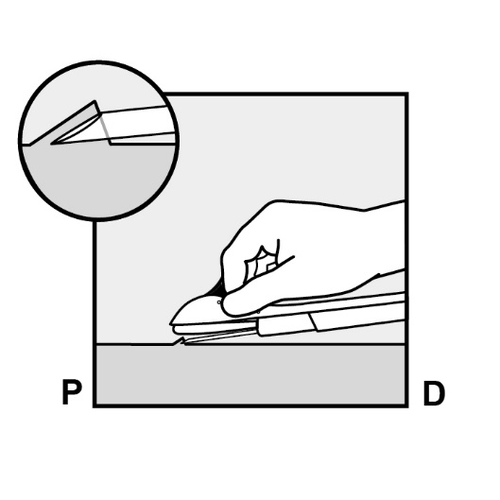
Figure 5a
Figure 5b
- Lower the applicator to a nearly horizontal position.
To facilitate the insertion of the implant under the skin, lift the skin with the needle, gently inserting the needle to its full length (Figure 6).
A slight resistance may be felt, but do not apply force.
If the needle is not inserted to its full length, the implant will not be properly inserted.
If the needle tip exits the skin before the needle is fully inserted, withdraw it and adjust its position under the skin to complete the insertion procedure.
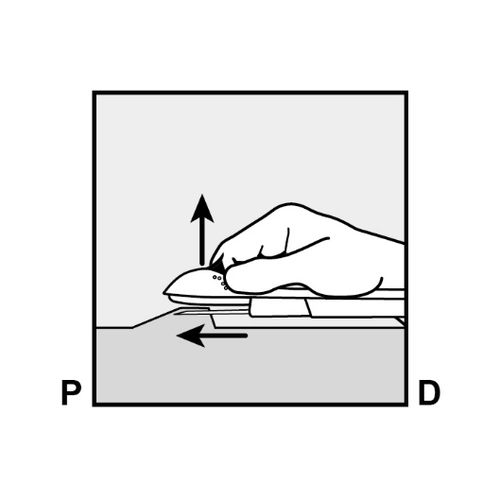
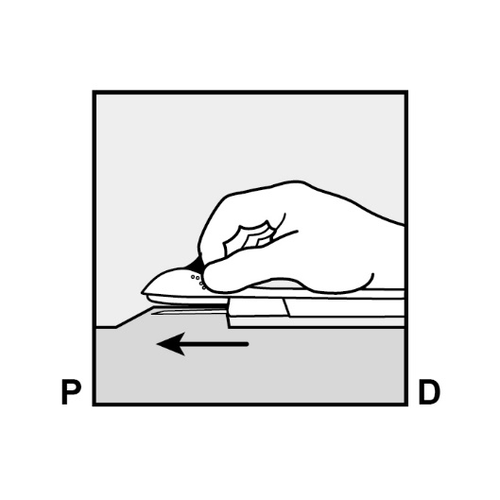
Figure 6
Figure 7
- Hold the applicator parallel to the skin surface and insert the needle to its full length (Figure 7).
If necessary, stabilize the applicator with the free hand. - To release the purple slider, gently push it downward (Figure 8a).

Push the slider back until it stops.
Do not move the applicator during the sliding of the purple slider(Figure 8b).
The implant is now under the skin, and the needle is locked inside the applicator.
The applicator can now be removed (Figure 8c).
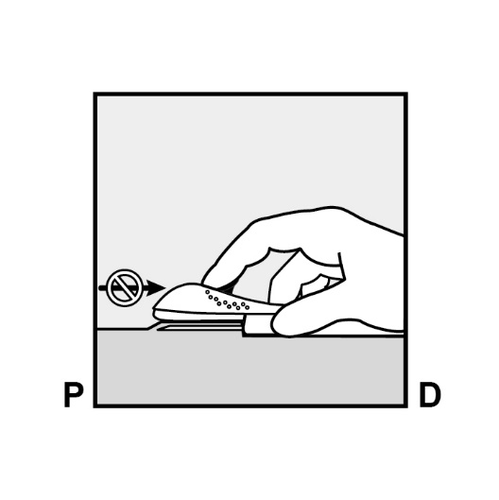
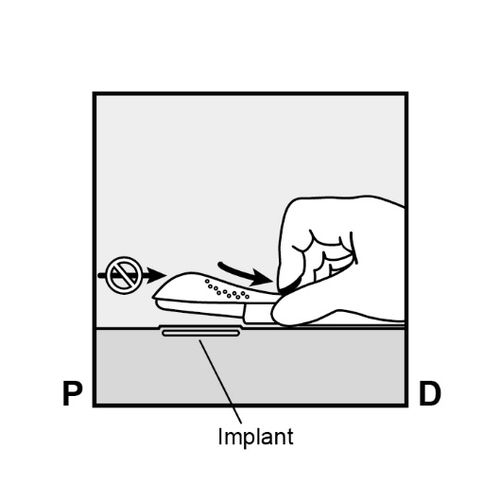
Figure 8a
Figure 8b
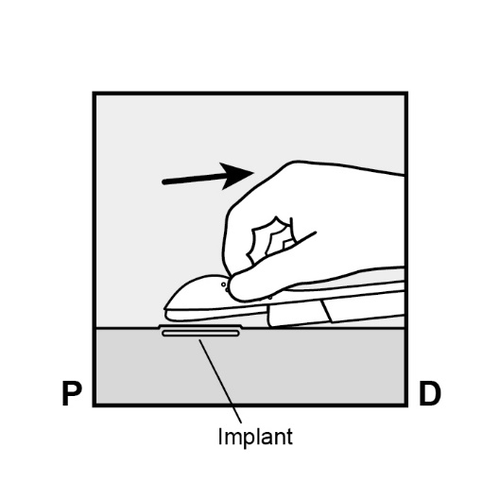
Figure 8c
If the applicator is not held in the same position during the entire procedure or if the purple slider is not pushed back to the stop, the implant will not be properly inserted and may protrude from the insertion site.
If the implant protrudes from the insertion site, it should be removed and a new insertion procedure should be performed at the same site using a new applicator.
Do not push the protruding implant back into the incision.
- Apply a small adhesive bandage to the implant insertion site.
- Always verify the presence of the implant by palpation immediately after insertion.
We should be able to palpate both ends of the 4 cm implant (Figure 9).
See below for "If the implant cannot be palpated after insertion".
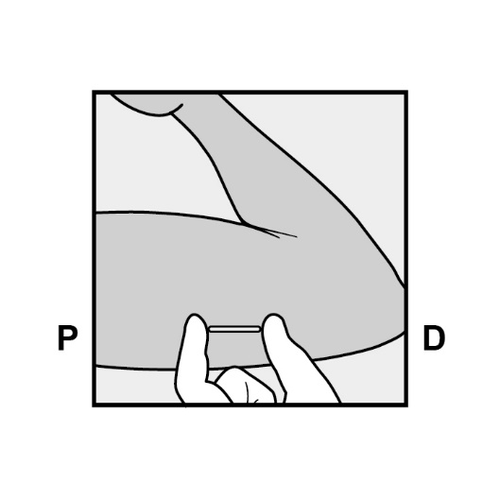
Figure 9
- Instruct the patient to palpate the implant regularly.
- Apply a sterile gauze and a pressure bandage to prevent hematoma formation at the implant insertion site.
The patient can remove the pressure bandage after 24 hours and the adhesive gauze after 3-5 days after implant insertion. - Complete the Patient Alert Card and give it to the patient.
Also, complete the label and attach it to the patient's chart.
If electronic patient charts are used, the information from the label should also be included. - The applicator is for single use and should be disposed of properly, according to local regulations for handling hazardous waste.
If the implant cannot be palpated after insertion:
In the event that the implant cannot be palpated or if there is doubt about its insertion, this may indicate that the implant was not inserted or was inserted too deeply:
- Check the applicator.
The needle should be fully retracted, and only the purple tip of the obturator should be visible. - Use other methods to confirm the presence of the implant.
Since the implant is not radiopaque, suitable localization methods include two-dimensional X-ray and computed tomography (CT).
Ultrasound (USS) with a high-frequency linear probe (10 MHz or higher) or magnetic resonance imaging (MRI) can be used.
If the implant cannot be palpated using the above imaging methods, measure the etonogestrel level in the patient's blood.
Consult the manufacturer for further instructions. - Until the presence of the implant is confirmed, the patient should use a non-hormonal contraceptive method.
- Deeply inserted implants should be localized and removed as soon as possible to avoid potential migration to a distant site (see section 4.4 of the Summary of Product Characteristics).
7.3 How to Remove Implanon NXT
The implant can be removed in aseptic conditions by a trained healthcare provider who is familiar with the removal technique.
Persons not experienced in the removal technique should consult the manufacturer for further information.
Before starting the removal procedure, the healthcare provider should assess the implant location.
The implant should be localized by palpation in the arm.
For removal of a palpable implant:
- Place the patient on her back on the examination table with her arm bent at the elbow and her hand under her head (or in a similar position) (Figure 10).
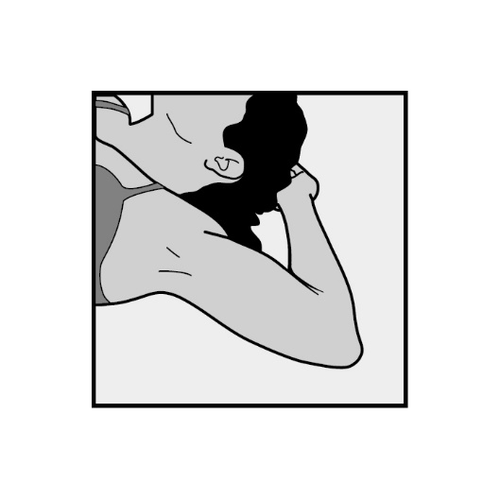
Figure 10
- Localize the implant by palpation.
Push the end of the implant closest to the shoulder downward (Figure 11) to stabilize it;
a bulge should appear, indicating the implant end closest to the elbow.
If the end does not bulge upward, removal of the implant may be difficultand should be performed by a healthcare provider experienced in removing deeply inserted implants.
Consult the manufacturer for further information. - Mark the distal end (the end closer to the elbow) with a surgical marker.
- Clean the area with an antiseptic solution.
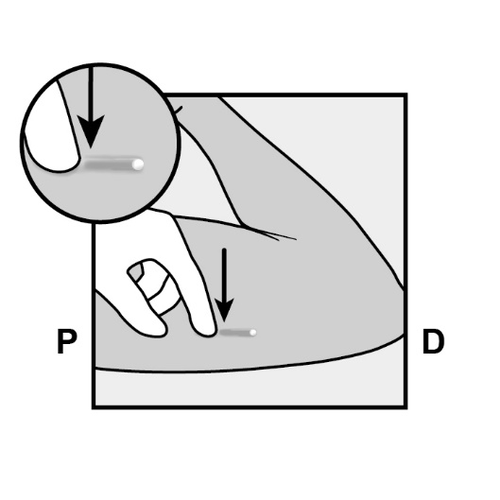
Figure 11
P, proximally (toward the shoulder);
D, distally (toward the elbow)
- Anesthetize the planned incision site, e.g., by injecting 0.5 to 1 ml of 1% lidocaine solution (Figure 12).
Administer the anesthetic underthe implant, so that the implant is close to the skin surface.
Injecting the anesthetic above the implant may make its removal more difficult.
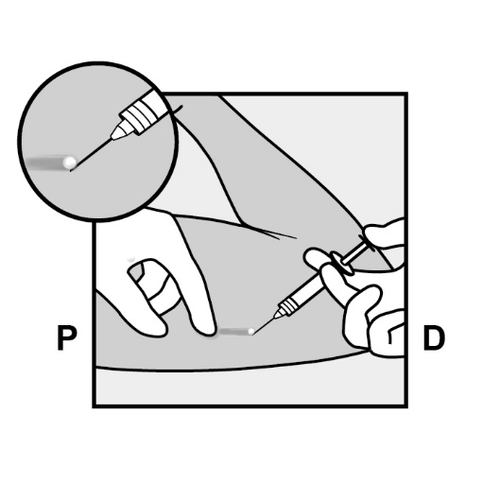
Figure 12
- Push the end of the implant closest to the shoulder downward (Figure 13) to stabilize it during the procedure.
Make a 2 mm longitudinal incision (parallel to the implant) in the skin, starting from the site above the implant end closest to the elbow.
Be careful not to cut the implant end.
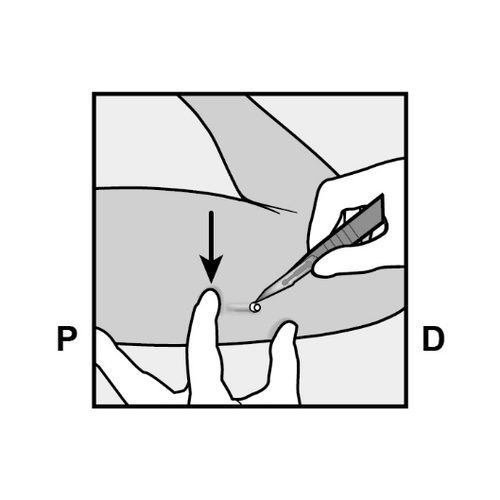
Figure 13
- The implant end should appear from the incision.
If it does not, gently push the implant in the direction of the incision until its end is visible.
Grasp the implant with forceps and remove it, if possible (Figure 14).
If necessary, gently dissect the tissue that has grown around the implant end, separating it bluntly.
If the implant end is still not exposed after dissecting the tissue, incise the tissue sheath and then remove the implant with forceps (Figures 15 and 16).
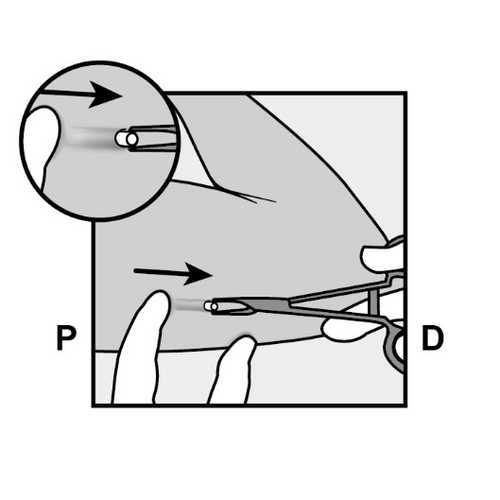
Figure 14
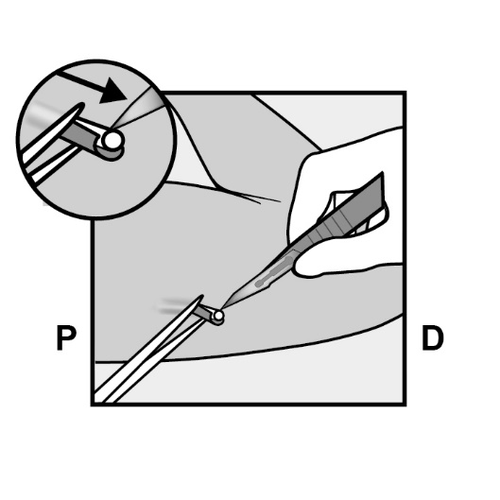
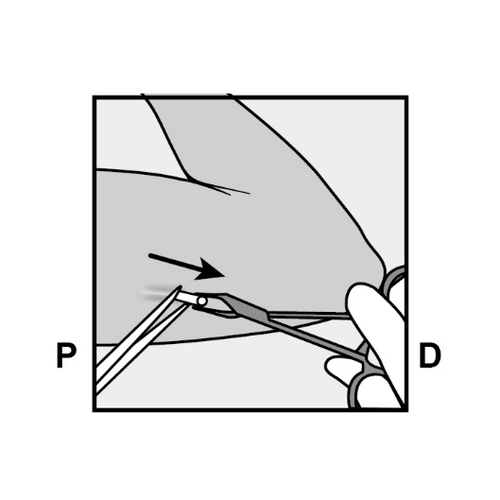
Figure 15
Figure 16
- If the implant is not visible, gently insert the forceps (preferably curved vascular forceps with the tips pointing upward) superficially into the incision site (Figure 17).
- Gently grasp the implant and then transfer the forceps to the other hand (Figure 18).
- Using other forceps, carefully dissect the tissue surrounding the implant (Figure 19).
The implant can then be removed. - If the implant cannot be grasped, the procedure should be discontinued, and the patient should be referred to a healthcare provider experienced in removing complicated implants or consult the manufacturer.
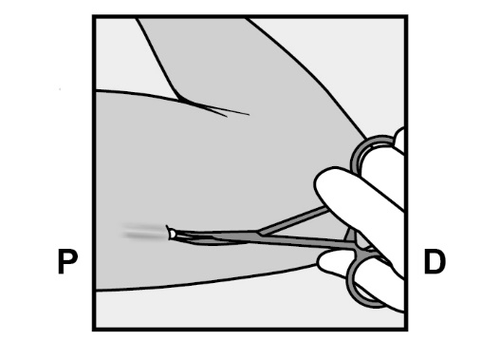
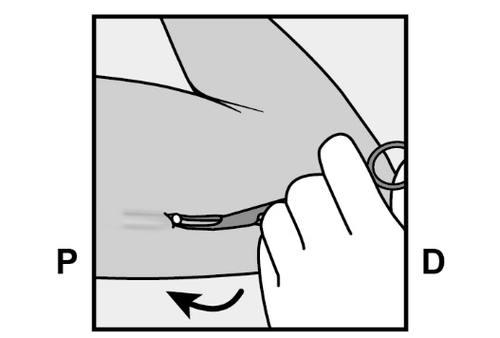
Figure 17
Figure 18
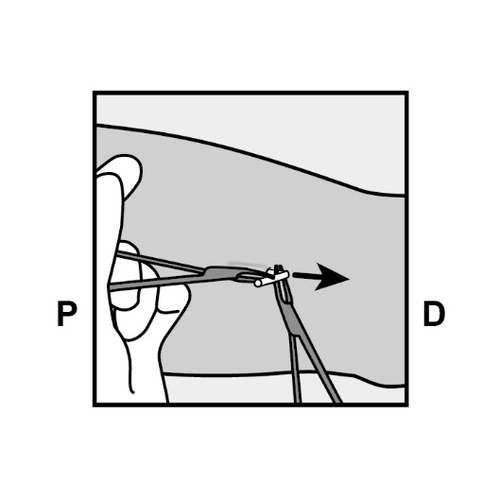
Figure 19
- Verify that the entire 4 cm implant has been removed by measuring its length.
There have been reports of implant breakage in the patient's arm.
In some cases, it has been difficult to remove the broken implant.
If only part of the implant is removed (less than 4 cm), the remaining fragment should be removed, following the instructions in this section. - If the patient wishes to continue using the Implanon NXT implant, a new implant can be inserted immediately after removal of the previous one, as described in section 7.2.
- After removing the implant, close the incision site with sterile wound closure strips.
- Apply a sterile gauze and a pressure bandage to prevent hematoma formation.
The patient can remove the pressure bandage after 24 hours and the sterile strips after 3-5 days after implant removal.
Localization and removal of a non-palpable implant:
In rare cases, implant migration has been reported;
usually, this is a minor migration relative to the original location (see also section 4.4 of the Summary of Product Characteristics),
but it may make detection of the original implant location by palpation impossible.
An implant inserted too deeply or migrated may not be palpable and therefore, as described below, imaging techniques may be required for its localization.
Before attempting to remove a non-palpable implant, it should always be localized first.
Since the implant is not radiopaque, suitable localization methods include two-dimensional X-ray and computed tomography (CT).
Ultrasound (USS) with a high-frequency linear probe (10 MHz or higher) or magnetic resonance imaging (MRI) can be used.
After localizing the implant in the arm, it should be removed by a healthcare provider experienced in removing deeply inserted implants and familiar with the arm's anatomy.
Consider using ultrasound during the implant removal procedure.
If the implant cannot be localized in the arm after multiple attempts, consider using imaging methods of the chest, as in extremely rare cases, implant migration to the pulmonary vessels has been reported.
If the implant is localized in the chest, its removal may require a surgical procedure or interventional methods;
consult a healthcare provider familiar with chest anatomy.
If these imaging methods fail to localize the implant, measure the etonogestrel level in the patient's blood.
Consult the manufacturer for further instructions.
Non-palpable and deeply inserted implants should be removed by a healthcare provider familiar with the arm's anatomy and the procedure for removing deeply inserted implants.
Exploratory surgery without prior localization of the implant is not recommended.
Consult the manufacturer for further instructions.
7.4 How to Replace Implanon NXT
After removing the previous implant, a new one can be inserted immediately, as described in section 7.2.
A new implant can be inserted in the same arm and often through the same incision used for removal of the previous implant,
provided that the incision is correctly located, i.e., 8-10 cm from the medial epicondyle of the humerus and 3-5 cm posteriorly (below) from the groove between the biceps and triceps muscles (see section 4.2 "How to Insert Implanon NXT" of the Summary of Product Characteristics).
- If the same incision is used, anesthetize the implant insertion site by injecting an anesthetic (e.g., 2 ml of 1% lidocaine solution) just under the skin, close to the incision, along the "implant insertion channel", and follow the instructions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterN.V. Organon
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Implanon NxtDosage form: Tablets, 75 mcgActive substance: desogestrelManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: Tablets, 75 microgramsActive substance: desogestrelPrescription requiredDosage form: Tablets, 75 microgramsActive substance: desogestrelPrescription required
Alternatives to Implanon Nxt in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Implanon Nxt in Spain
Online doctors for Implanon Nxt
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Implanon Nxt – subject to medical assessment and local rules.