
Imikeraderm

Ask a doctor about a prescription for Imikeraderm

How to use Imikeraderm
Patient Information Leaflet: User Information
Imikeraderm, 50 mg/g, cream
Imiquimod
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Imikeraderm and what is it used for
- 2. Important information before using Imikeraderm
- 3. How to use Imikeraderm
- 4. Possible side effects
- 5. How to store Imikeraderm
- 6. Contents of the pack and other information
1. What is Imikeraderm and what is it used for
Imikeraderm is used to treat actinic keratosis.
Actinic keratosis is a condition characterized by the appearance of rough patches on the skin of people who have been exposed to the sun for a long time. Some skin changes are skin-colored, while others may be grayish, pink, red, or brown. These changes can be flat and scaly or raised, rough, hard, and wart-like. Imikeraderm can only be used on flat actinic keratosis on the face and balding scalp, in patients with a normally functioning immune system, if the doctor considers that treatment with Imikeraderm cream is the most suitable for the patient.
Imikeraderm helps the body's immune system produce natural substances that help fight actinic keratosis.
2. Important information before using Imikeraderm
When not to use Imikeraderm:
Warnings and precautions
Before starting treatment with Imikeraderm, discuss it with your doctor or pharmacist.
- do not use Imikeraderm until the treated area has healed after previous treatment with medicines or surgery.
- avoid contact with eyes, mouth, and nostrils. In case of accidental contact, remove the cream by rinsing with water.
- do not use the cream internally.
- do not use more cream than prescribed by your doctor.
- do not cover the treated area with bandages or other dressings after applying Imikeraderm.
During treatment with Imikeraderm, do not use sunbeds and, if possible, avoid sunlight. When outdoors, wear protective clothing and a hat with a wide brim.
During treatment with Imikeraderm, until the skin changes have healed, the treated area may differ significantly from normal skin.
Children and adolescents
It is not recommended to use this medicine in children and adolescents.
Imikeraderm and other medicines
tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take.
there are no known medicines that interact with Imikeraderm.
Pregnancy and breastfeeding
do not breastfeed while using Imikeraderm, as it is not known whether imiquimod is excreted in human milk.
Driving and using machines
this medicine has no or negligible influence on the ability to drive and use machines.
Imikeraderm contains methyl parahydroxybenzoate, propyl parahydroxybenzoate (E 218 and E 216)
methyl parahydroxybenzoate (E 218) and propyl parahydroxybenzoate (E 216) may cause allergic reactions (possible late reactions).
Imikeraderm contains cetyl alcohol and stearyl alcohol
cetyl alcohol and stearyl alcohol may cause local skin reactions (e.g., contact dermatitis).
Imikeraderm contains benzyl alcohol
the medicine contains 5 mg of benzyl alcohol in each sachet. Benzyl alcohol may cause allergic reactions and mild local irritation.
Imikeraderm contains butylhydroxytoluene (E 321)
butylhydroxytoluene (E 321) may cause local skin reactions (e.g., contact dermatitis) or eye and mucous membrane irritation.
3. How to use Imikeraderm
Children and adolescents:
it is not recommended to use this medicine in children and adolescents.
Adults:
always use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
before and after applying the cream, wash your hands thoroughly. Do not cover the treated area with bandages or other dressings after applying Imikeraderm.
each time you use the cream, use a new sachet. After using the cream from the sachet, remove the sachet with the remaining cream. Do not leave an open sachet for later use.
Imikeraderm - instructions for use
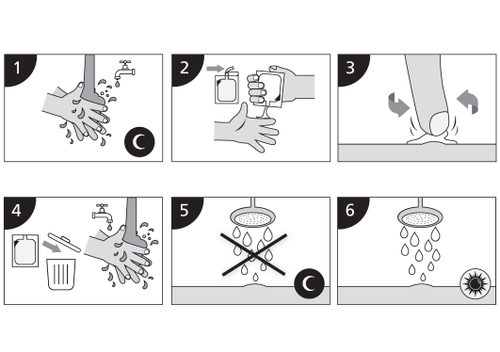
Instructions for use (Monday, Wednesday, and Friday)
- 1. Before going to bed, wash your hands and the treated area with water and mild soap. Dry thoroughly.
- 2. Open a new sachet and squeeze the cream onto the tip of your finger.
- 3. Apply a thin layer of cream to the affected area. Rub gently until the cream has completely penetrated the skin.
- 4. After applying the cream, remove the open sachet. Wash your hands with water and soap.
- 5. Leave Imikeraderm on the skin for about 8 hours. During this time, do not shower or bathe.
- 6. After about 8 hours, wash the area where you applied Imikeraderm with water and mild soap.
Imikeraderm should be applied 3 times a week, for example on Monday, Wednesday, and Friday. One sachet contains enough cream to cover an area of about 25 cm². Continue treatment for 4 weeks. After the first 4 weeks of treatment, your doctor will assess the condition of your skin. If the changes have not disappeared, it may be necessary to extend the treatment by another 4 weeks.
If you use more Imikeraderm than you should
wash off the excess with water and mild soap. After the skin reaction has subsided, you can restart the treatment.
if you accidentally swallow Imikeraderm, contact your doctor.
If you forget to use Imikeraderm
4. Possible side effects
like all medicines, Imikeraderm can cause side effects, although not everybody gets them.
if you experience any side effects during treatment with Imikeraderm, tell your doctor or pharmacist as soon as possible.
some patients have experienced skin discoloration at the site of application of Imikeraderm.
although these changes usually disappear over time, in some patients they may be permanent. If your skin reacts badly during treatment with Imikeraderm, stop using the cream, wash the treated area with water and mild soap, and contact your doctor or pharmacist.
some patients have experienced a decrease in blood cell count. A decrease in blood cell count may increase the risk of infection, cause more frequent bruising, or cause fatigue. If you experience such symptoms, tell your doctor.
in some patients with autoimmune disorders, their condition may worsen. If you notice any changes during treatment with Imikeraderm, tell your doctor.
rarely, serious skin reactions have been reported. If you experience skin changes or spots on the skin, initially as small red spots, and then as small dots, which may be accompanied by symptoms such as itching, fever, general malaise, joint pain, eye problems, burning, pain, or itching of the eyes, and mouth ulcers, stop using Imikeraderm and inform your doctor.
a small number of patients have experienced hair loss at the site of application or in its vicinity.
the cause of many side effects of Imikeraderm is local action on the skin. Local skin reactions may indicate that the medicine is working as intended.
Very common (may affect more than 1 in 10 people)
very commonside effects include mild itching in the treated skin area.
Common (may affect up to 1 in 10 people)
commonside effects include pain, burning sensation, irritation, or redness.
Uncommon (may affect up to 1 in 100 people)
uncommonside effects have been reported in some patients, including changes at the site of application (bleeding, inflammatory reaction, discharge, hypersensitivity, swelling, swelling of small areas of skin, tingling, flaking, scarring, ulceration, or feelings of warmth or discomfort) or inflammatory reactions of the mucous membrane lining the nose, nasal congestion, flu-like symptoms, depression, eye irritation, swelling of the eyelids, sore throat, diarrhea, actinic keratosis, redness, swelling of the face, ulcers, limb pain, fever, weakness, or chills.
Reporting side effects
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
side effects can also be reported to the marketing authorization holder.
by reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Imikeraderm
keep the medicine out of the sight and reach of children.
do not store above 25°C.
do not use this medicine after the expiry date stated on the packaging and sachet after "EXP".
the expiry date refers to the last day of the month.
do not reuse the cream from an open sachet.
medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Imikeraderm contains
the active substance is:
imiquimod.
each sachet contains 250 mg of cream (100 mg of cream contains 5 mg of imiquimod).
the other ingredients are:
isostearic acid, benzyl alcohol, white petrolatum (stabilized with butylhydroxytoluene E 321), cetyl alcohol, stearyl alcohol, polysorbate 60, sorbitan stearate (type I), glycerol, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), xanthan gum, purified water (see also section 2).
What Imikeraderm looks like and contents of the pack
each Imikeraderm sachet contains 250 mg of cream, white to light yellow in color. Each pack contains 12 single-use sachets made of PET/LDPE/Aluminum/Surlyn.
not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
SUN-FARM Sp. z o.o.
Dolna 21
05-092 Łomianki
phone: +48 22 350 66 69
Manufacturer
mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
SUN-FARM Sp. z o.o.
Dolna 21
05-092 Łomianki
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany:
Imikeraderm 50 mg/g Creme
Austria:
Imikeraderm 50 mg/g Creme
Poland:
Imikeraderm
Spain:
Imikeraderm 50 mg/g crema
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- Importermibe GmbH Arzneimittel Sun-Farm Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ImikeradermDosage form: Cream, 50 mg/gActive substance: aciclovirManufacturer: Ziaja Ltd. - Zakład Produkcji Leków Sp. z o.o.Prescription not requiredDosage form: Cream, 50 mg/gActive substance: aciclovirManufacturer: Medinfar Manufacturing S.A.Prescription not requiredDosage form: Solution, 5 mg/mlActive substance: podophyllotoxinManufacturer: Delpharm Bladel B.V.Prescription required
Alternatives to Imikeraderm in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Imikeraderm in Spain
Alternative to Imikeraderm in Ukraine
Online doctors for Imikeraderm
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Imikeraderm – subject to medical assessment and local rules.







