
IMIKERADERM 50 mg/g CREAM

How to use IMIKERADERM 50 mg/g CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Imikeraderm 50 mg/g Cream
Imiquimod
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Imikeraderm and what is it used for
- What you need to know before you start using Imikeraderm
- How to use Imikeraderm
- Possible side effects
- Storage of Imikeraderm
- Contents of the pack and other information
1. What is Imikeraderm and what is it used for
Imikeraderm is indicated for the treatment of actinic keratosis.
Actinic keratosis consists of rough areas of the skin found in people who have been exposed to a lot of sunlight during their lifetime. Some are colored skin, others are grayish, pink, red or brown. They can be smooth and scaly, or thickened, hard and wart-like. Imikeraderm should be used exclusively for smooth actinic keratosis on the face and scalp of patients with a healthy immune system when your doctor decides that Imikeraderm is the most appropriate treatment.
Imikeraderm helps the body's own immune system to produce natural substances that help fight actinic keratosis.
2. What you need to know before you start using Imikeraderm
Do not use Imikeraderm
Warnings and precautions
Consult your doctor or pharmacist before starting to use imiquimod cream.
- If you have used imiquimod cream or similar preparations before, you should consult your doctor before starting this treatment.
- If you have autoimmune disorders.
- If you have had an organ transplant.
- Do not use imiquimod cream until the area to be treated has healed after previous pharmacological or surgical treatment.
- Avoid contact with the eyes, lips, and nasal passages. In case of accidental contact, remove the cream by washing with water.
- Do not apply the cream internally.
- Do not use more cream than your doctor advises.
- Do not cover the treated area with bandages or other dressings after applying imiquimod cream.
- If the treated area becomes too irritated, the cream should be removed with mild soap and water.
As soon as the problem is resolved, you can reapply the cream.
- Tell your doctor if you have changes in your blood count.
Due to the mode of action of imiquimod cream, there is a possibility that the cream may worsen existing inflammation in the treatment area.
Do not use sunlamps or tanning devices and avoid sunlight as much as possible during treatment with imiquimod cream. When going out, use protective clothing and wide-brimmed hats.
During the use of imiquimod cream and until healing, it is likely that the treatment area will have a noticeably different appearance from normal skin.
Children and adolescents
It is not recommended for use in children and adolescents.
Other medicines and Imikeraderm
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
No medicines are known to be incompatible with imiquimod cream.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
You should tell your doctor if you are pregnant or plan to become pregnant. He will assess the risks and benefits of using imiquimod cream during pregnancy. Animal studies do not indicate direct or indirect harmful effects during pregnancy.
Do not breastfeed while using imiquimod cream, as it is not known whether imiquimod is excreted in breast milk.
Driving and using machines
This medicine has a negligible influence on the ability to drive and use machines.
Imikeraderm contains methylparaben and propylparaben (E218 and E216)
Methylparaben (E218) and propylparaben (E216) may cause allergic reactions (possibly delayed).
Imikeraderm contains cetyl alcohol and stearic alcohol
Cetyl alcohol and stearic alcohol may cause local skin reactions (such as contact dermatitis).
Imikeraderm contains benzyl alcohol
This medicine contains 5 mg of benzyl alcohol in each sachet. Benzyl alcohol may cause allergic reactions and moderate local irritation.
Imikeraderm contains butylhydroxytoluene (E321)
Butylhydroxytoluene (E321) may cause local skin reactions (such as contact dermatitis) or eye and mucous membrane irritation.
3. How to use Imikeraderm
Children and adolescents:
It is not recommended for use in children and adolescents.
Adults:
Follow your doctor's instructions for administering this medicine exactly. If in doubt, consult your doctor or pharmacist again.
Wash your hands carefully before and after applying the cream. Do not cover the treated area with bandages or other dressings after applying imiquimod cream.
Open a new sachet each time you apply the cream. Discard any remaining cream in the sachet after application. Do not store the opened sachet for use on another day.
Instructions for applying Imikeraderm
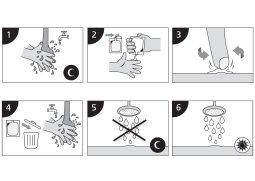
Application instructions: - (Monday, Wednesday, and Friday)
- Before bedtime, wash your hands and the treatment area with mild soap and water. Dry well.
- Open a new sachet and place a small amount of cream on the tip of your finger.
- Apply imiquimod cream to the affected area. Spread it gently over the area until the cream disappears.
- After applying the cream, discard the opened sachet and wash your hands with water and soap.
- Let imiquimod cream act on the skin for about 8 hours. During this time, do not shower or bathe.
- After about 8 hours, wash the area where you applied imiquimod cream with mild soap and water.
Apply imiquimod cream 3 times a week. For example, apply the cream on Mondays, Wednesdays, and Fridays. One sachet contains enough cream to cover an area of 25 cm2. Continue treatment for 4 weeks. Four weeks after finishing the first treatment, your doctor will assess your skin. If not all lesions have disappeared, another 4 weeks of treatment may be necessary.
If you use more Imikeraderm than you should
Remove the excess with water and mild soap. When the skin reaction disappears, you can continue treatment.
In case of accidental ingestion of imiquimod, consult your doctor.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Imikeraderm
If you forget a dose, apply the cream as soon as possible and continue with the usual schedule.
Do not apply the cream more than once a day.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Consult your doctor or pharmacist if you do not feel well during the administration of imiquimod.
Some patients have presented with skin color changes in the area where imiquimod was applied. Although these changes tend to improve over time, they could be permanent in some patients. If your skin has an adverse reaction to using imiquimod cream, interrupt the application of the cream, wash the area with water and mild soap, and contact your doctor or pharmacist.
In some individuals, a decrease in blood counts has been detected. A decrease in blood counts can make you more susceptible to infections, cause you to bruise more easily, or cause fatigue. If you notice any of these symptoms, tell your doctor.
Some patients with autoimmune disorders may experience worsening of their disease. Consult your doctor if you experience any changes during treatment with imiquimod cream.
In rare cases, severe skin reactions have occurred. Interrupt treatment with imiquimod cream and inform your doctor immediately if you notice skin lesions or spots on the skin that start as small red areas and evolve into small targets, possibly with inflammation, fever, feeling of general discomfort, vision problems, burning, swollen or painful eyes, and swollen mouth.
A small number of patients have experienced hair loss in the treated area or surrounding area.
Many of the side effects of imiquimod cream are due to local action on your skin. Local skin reactions can be a sign that the drug is working as expected.
Very common (observed in more than 1 in 10 patients).
Very oftenthe treated skin may present with mild itching.
Common (observed in less than 1 in 10 patients).
Common effectsinclude: pain, burning, irritation, or redness.
If any skin reaction becomes too bothersome during treatment, consult your doctor. He may advise you to stop applying imiquimod cream for a few days (i.e., a brief break from treatment). If pus or other signs of infection appear, inform your doctor. Apart from skin reactions, other common effects are headache, anorexia, nausea, muscle and joint pain, and fatigue.
Uncommon (observed in less than 1 in 100 patients).
Uncommonly, some patients experience changes at the application site (bleeding, inflammation, secretion, sensitivity, swelling, small inflamed areas on the skin, tingling, scaling, scarring, ulceration, or sensation of heat or discomfort), or inflammation of the skin covering the nose, nasal obstruction, flu or flu-like symptoms, depression, eye irritation, eyelid inflammation, sore throat, diarrhea, actinic keratosis, redness, facial swelling, ulcers, pain in a limb, fever, weakness, or tremors.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Imikeraderm
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Do not use this medicine after the expiry date stated on the outer packaging and on the label after EXP. The expiry date refers to the last day of that month.
Once opened, sachets should not be reused.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at your pharmacy. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Imikeraderm
The active substance is:
Imiquimod
Each sachet contains 250 mg of cream (100 mg of cream contains 5 mg of imiquimod).
The other ingredients are:
Isostearic acid, benzyl alcohol, white petrolatum (stabilized with butylhydroxytoluene E321), cetyl alcohol, stearic alcohol, polysorbate 60, sorbitan stearate type I, glycerol, methylparaben (E218), propylparaben (E216), xanthan gum, and purified water (see also section 2 "Imikeraderm 50 mg/g cream contains methylparaben, propylparaben, cetyl alcohol, stearic alcohol, benzyl alcohol, and butylhydroxytoluene").
Appearance of the product and pack contents
Each Imikeraderm sachet contains 250 mg of a white or yellowish cream. Each pack contains 12 or 24 single-use polyester/paper/aluminum sachets.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Mibe Pharma España S.L.U.
C/Amaltea 9, 4th floor, letter B,
28045, Madrid
Spain
Manufacturer
Mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
or
Sun-Farm Sp. z o.o.
Ul. Dolna 21, Lomianki
05-092 Mazowieckie
Poland
Date of last revision of this leaflet: May 2022
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Average pharmacy price37.78 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IMIKERADERM 50 mg/g CREAMDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: CREAM, 50 mg/gActive substance: imiquimodManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for IMIKERADERM 50 mg/g CREAM
Discuss questions about IMIKERADERM 50 mg/g CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








