
Ig Vena

Ask a doctor about a prescription for Ig Vena

How to use Ig Vena
Package Leaflet: Information for the User
Ig VENA, 50 g/l, Solution for Infusion
Human Normal Immunoglobulin for Intravenous Administration (IVIg)
You should carefully read the contents of this leaflet before using this medicine, as it contains important information for you.
- You should keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Table of Contents of the Package Leaflet:
- 1. What is Ig VENA and what is it used for
- 2. Important information before using Ig VENA
- 3. How to use Ig VENA
- 4. Possible side effects
- 5. How to store Ig VENA
- 6. Contents of the pack and other information
1. What is Ig VENA and what is it used for
Ig VENA is a solution of human normal immunoglobulin for intravenous administration.
Immunoglobulins are human antibodies that are also present in the blood.
Ig VENA is used in the following therapies:
- 1. Treatment of adults and children and adolescents (0-18 years) when the patient does not have enough antibodies (replacement therapy) in the following cases:
- 2. In patients with congenital antibody deficiency (primary immunodeficiency syndromes).
- 3. In patients with acquired antibody deficiency (secondary immunodeficiency), who experience severe or recurrent infections caused by various clinical conditions (e.g., cancer or autoimmune diseases, or as a result of treatment of these diseases). Antibiotic treatment in these patients was ineffective, and they either did not achieve a sufficient increase in IgG antibody levels after vaccination (pneumococcal polysaccharide vaccine and vaccine containing a polypeptide antigen), or their IgG level in serum was <4 g l.< li>
Treatment of adults and children and adolescents (0-18 years) with certain inflammatory diseases (immunomodulation) in the following cases:
- 1. In patients with a low platelet count (primary immune thrombocytopenia) and in patients with a high risk of bleeding or before surgery to achieve an adequate platelet count.
- 2. In patients with Guillain-Barré syndrome. This is an acute disease characterized by inflammation of the peripheral nerves, leading to severe muscle weakness, mainly in the legs and arms.
- 3. In patients with Kawasaki disease (in combination with acetylsalicylic acid). Kawasaki disease is an acute disease of young children characterized by inflammation of blood vessels throughout the body.
- 4. In patients with chronic inflammatory demyelinating polyneuropathy (CIDP). This chronic disease is a rare disorder of the peripheral nerves, characterized by a gradually progressing weakness of the lower and, to a lesser extent, upper limbs.
- 5. In multifocal motor neuropathy (MMN). This is a rare disease that affects motor nerves and is characterized by a slow, progressive, asymmetric weakness of the limbs without sensory loss.
2. Important information before using Ig VENA
When not to use Ig VENA
If you are allergic (hypersensitive) to human normal immunoglobulin or any of the other ingredients of this medicine (listed in section 6).
If you have antibodies against immunoglobulin A (IgA) in your blood, as administration of a medicine containing IgA may lead to a severe allergic reaction.
Warnings and precautions
Before starting treatment with Ig VENA, you should discuss it with your doctor or nurse.
Patients must be closely monitored and carefully observed during infusion due to the risk of side effects.
Some side effects may occur more frequently:
- in case of too rapid infusion;
- in patients with untreated infection (e.g., fever) or chronic inflammatory condition;
- in patients receiving human normal immunoglobulin for the first time;
- in rare cases, when the previously administered human normal immunoglobulin product is changed to another or when a long time has passed since the last infusion.
In certain cases, immunoglobulins may increase the risk of heart attack, stroke, pulmonary embolism, or worsen deep vein thrombosis
For this reason, your doctor will exercise particular caution in the following cases:
- in obese patients,
- in elderly patients,
- in patients with diabetes,
- in patients with hypertension,
- in patients with reduced blood volume (hypovolemia),
- in patients with vascular diseases,
- in patients at risk of developing blood clotting disorders (acquired or congenital coagulation disorders),
- in patients with a history of blood clots,
- in patients with conditions characterized by increased blood viscosity,
- in patients who are immobilized for a long time,
- in patients with kidney disease, currently or in the past, or taking medications that may harm the kidneys (nephrotoxic drugs), as cases of acute kidney failure have been reported. In case of kidney damage, consideration should be given to discontinuing immunoglobulin administration.
The patient may be allergic (hypersensitive) to immunoglobulin (antibodies) without knowing it
Hypersensitivity may occur even in a patient who has previously received human normal immunoglobulin and tolerated it well. It may occur especially in cases of IgA deficiency (in patients with anti-IgA antibodies). In these rare cases, allergic reactions (hypersensitivity) such as a sudden drop in blood pressure or anaphylactic reaction may occur.
In case of a side effect, the infusion rate should be either reduced or the immunoglobulin administration should be discontinued. The treatment depends on the type and severity of the side effect. In case of anaphylactic shock, you should proceed according to the currently applicable medical standards for anaphylactic shock treatment. You should tell your doctor if any of the above cases apply to you. Your doctor will take the appropriate precautions when administering Ig VENA.
Prevention of viral infections
Medicines made from human blood or plasma are subject to certain procedures that are intended to prevent the transmission of infectious diseases to patients. These procedures include the selection of blood and plasma donors, whose purpose is to exclude donors who may be a source of infection; testing of plasma for the presence of infectious agents/viruses. Manufacturers of medicines from human blood or plasma also use processes that inactivate or remove viruses. Despite these preventive measures, it cannot be completely excluded that the transmission of infectious agents may occur through a medicine prepared from human blood or plasma. This also applies to unknown or newly discovered viruses and other pathogens.
It is considered that the preventive measures taken are effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), as well as against non-enveloped viruses, such as hepatitis A virus (HAV).
These measures may have limited effectiveness against non-enveloped viruses, such as parvovirus B19.
No evidence has been found that immunoglobulins can cause hepatitis A or parvovirus B19 infection, as the presence of antibodies may play a protective role against viral infections.
It is recommended that every time Ig VENA is administered, the name and batch number should be recorded to enable identification of the medicine.
Children and adolescents
After administration of Ig VENA in children and adolescents, transient and mild glucosuria (glucose in the urine) has been observed, without clinical symptoms. This may be related to the maltose content in Ig VENA, which is hydrolyzed to glucose in the renal tubules. Glucose is reabsorbed and excreted in the urine to a very small extent. Glucose reabsorption depends on the patient's age. Transient increased maltose levels in serum may exceed the renal threshold for glucose reabsorption and affect the results of glucose testing in the urine.
Ig VENA and other medicines
You should tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Human normal immunoglobulin for intravenous administration should not be mixed with other medicines or other immunoglobulin products for intravenous administration (IVIg).
Effect on live attenuated viral vaccines
Administration of immunoglobulin may weaken the effect of live attenuated viral vaccines, such as measles, rubella, mumps, or chickenpox vaccines, for a period of at least 6 weeks to 3 months. After administration of this medicine, a 3-month interval should be observed before vaccination with a live attenuated viral vaccine. In the case of measles, this weakening may last up to one year. Therefore, patients receiving measles vaccine should have their antibody titers determined.
Loop diuretics (a group of medicines that increase urine flow)
Concomitant use with loop diuretics should be avoided.
Effect on blood test results
- After injection of immunoglobulin, a transient increase in the blood of passively transferred various antibodies may cause false-positive results in serological tests.
- Passive transfer of antibodies against erythrocyte antigens, e.g., A, B, D (responsible for blood group), may affect the results of some serological tests for red blood cell antibodies, e.g., indirect antiglobulin test (DAT, Coombs test).
Blood glucose test
Some types of blood glucose tests (e.g., using methods based on glucose dehydrogenase - pyrroloquinolinequinone (GDH-PQQ) or glucose oxidoreductase - dye) may falsely interpret maltose (100 mg/ml) contained in Ig VENA as glucose. This may cause a falsely elevated reading of blood glucose levels during infusion and for about 15 hours after the end of infusion, which may lead to inappropriate administration of insulin, resulting in life-threatening hypoglycemia. Additionally, cases of actual hypoglycemia may remain untreated if the hypoglycemic state is masked by falsely elevated glucose readings.
In this regard, when administering Ig VENA or other parenteral medicines containing maltose, blood glucose measurement should be performed using a glucose-specific method. You should carefully read the information about glucose tests, including information about test strips, to determine if they can be used with parenteral medicines containing maltose. In case of doubt, you should contact the device manufacturer to determine if they can be used with parenteral medicines containing maltose.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor for advice before taking this medicine. Your doctor will decide whether Ig VENA can be used during pregnancy.
- No clinical studies have been conducted with Ig VENA in pregnant women. It has been shown that intravenous immunoglobulin products cross the placenta, with increased levels during the third trimester. However, long-term clinical experience with intravenous immunoglobulin suggests that no harmful effects on the course of pregnancy, the fetus, or the newborn are expected.
- If you are breastfeeding and receiving Ig VENA, antibodies from this medicine may pass into human milk. This may contribute to the protection of the newborn against certain infections.
- Clinical experience with intravenous immunoglobulin suggests that no harmful effects on fertility are expected.
Driving and using machines
Some side effects associated with Ig VENA may impair the ability to drive and use machines. Patients who experience side effects during treatment should wait for them to resolve before driving or operating machines.
Ig VENA contains maltose and sodium
The medicine contains 100 mg of maltose in 1 ml.
This medicine contains approximately 69 mg of sodium in 1 liter. This should be taken into account in patients on a low-sodium diet.
3. How to use Ig VENA
Ig VENA can only be administered by a doctor or trained medical staff in a hospital or outpatient setting.
The dose and dosing schedule depend on the indications; your doctor will determine the appropriate dosing for your individual needs.
Ig VENA should be administered initially slowly. If the medicine is well tolerated, the infusion rate can be gradually increased.
Use in children and adolescents
Dosing in children and adolescents (0-18 years) does not differ from that in adults, as dosing in individual indications is determined based on the patient's body weight and clinical condition.
Overdose of Ig VENA
Overdose may lead to circulatory overload and excessive blood viscosity, especially in patients at risk, elderly patients, or those with heart or kidney failure.
In case of any further questions about the use of this medicine, you should consult your doctor or nurse.
4. Possible side effects
Like all medicines, Ig VENA can cause side effects, although not everybody gets them.
The following side effects may occur after administration of a medicine containing immunoglobulins:
- chills, headache, dizziness, fever, vomiting, nausea, allergic reactions, joint pain, low blood pressure, and moderate lower back pain may occur occasionally;
- isolated cases of transient reduction in red blood cell count (reversible hemolytic anemia/hemolysis);
- sudden drop in blood pressure may occur rarely, and in isolated cases, anaphylactic shock may occur, even in patients who have not experienced hypersensitivity after previous administrations;
- rare cases of transient skin reactions have been observed;
- very rarely, thromboembolic complications (blood clots) have occurred, which can lead to heart attack, stroke, pulmonary embolism, or deep vein thrombosis;
- cases of transient, non-infectious meningitis (aseptic meningitis) have been observed;
- increased serum creatinine levels and/or acute kidney failure have been observed;
- cases of acute lung injury related to transfusion (TRALI - Transfusion-related acute lung injury) have been reported.
In clinical trials and after the marketing of Ig VENA, the following side effects have been observed, listed in order of decreasing frequency.
Common (may affect up to 1 in 10 people)
- Back pain
- Nausea
- Feeling weak, tired, feverish
- Muscle pain
- Headache, drowsiness
Frequency not known (cannot be estimated from the available data)
- Aseptic meningitis syndrome
- Red blood cell destruction causing anemia
- Allergic reactions and life-threatening anaphylactic shock
- Confusion
- Stroke, dizziness, uncontrolled trembling, numbness or tingling of the skin or limbs
- Heart attack, bluish discoloration of the skin, rapid heartbeat, slow heartbeat, irregular heartbeat
- Blood clots in deep veins and blood vessels, low blood pressure, high blood pressure, paleness
- Pulmonary embolism, fluid accumulation in the lungs, breathing difficulties with wheezing or cough
- Vomiting, diarrhea, abdominal pain
- Rapidly spreading skin swelling, hives, redness and inflammation of the skin, skin rash, itching, eczema, excessive sweating
- Joint and muscle pain, back pain, neck pain, stiffness of skeletal muscles
- Sudden kidney failure
- Phlebitis at the injection site, fever, pain or discomfort in the chest, facial swelling, general malaise
- Increased creatinine levels in the blood
Additional side effects in children and adolescents
You should expect the frequency, type, and severity of side effects in children and adolescents to be the same as in adults.
After administration of Ig VENA in children and adolescents, transient and mild glucosuria (glucose in the urine) has been observed, without clinical symptoms.
Information on viral safety can be found in section 2 "Important information before using Ig VENA".
Reporting side effects
If you experience any side effects, talk to your doctor or nurse. Side effects can be reported to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides (Al. Jerozolimskie 181C, 02-222 Warsaw),
phone: 22 4921301,
fax: 22 4921309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
5. How to store Ig VENA
Medicines should be kept out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after "EXP". The expiry date refers to the last day of that month.
Store in a refrigerator (2°C - 8°C).
Before use and during the shelf-life, the medicine can be stored at room temperature (not above 25°C) for a maximum of 6 consecutive months. After this time, the medicine should be discarded. In no case can the medicine be returned to the refrigerator if it has been stored at room temperature.
On the carton, you should record the start date of storage at room temperature.
After opening the vial, the contents should be used immediately.
Vials should be stored in the outer packaging. Do not freeze.
Do not use this medicine if you notice that the solution has become cloudy, has changed color, or has visible sediment.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Ig VENA contains
The active substance is human normal immunoglobulin.
1 ml of solution contains 50 mg of human normal immunoglobulin.
The solution contains human protein 50 g/l, including at least 95% IgG (immunoglobulin G).
The distribution of IgG subclasses is as follows:
IgG1 62.1%,
IgG2 34.8%,
IgG3 2.5%,
IgG4 0.6%.
The maximum IgA content is 50 micrograms/ml.
The medicine is produced from the plasma of blood donors.
The other ingredients are maltose, water for injections.
What Ig VENA looks like and contents of the pack
Ig VENA solution for infusion is available in single vials of 50 ml, 100 ml, or 200 ml with an installed handle (vial + handle). The solution is clear or slightly opalescent, colorless or pale yellow.
Package sizes:
Single packs:
1 vial containing 2.5 g/50 ml
1 vial containing 5 g/100 ml
1 vial containing 10 g/200 ml
Multi-packs:
Multi-pack containing 2 single packs of 1 vial of 10 g/200 ml
Multi-pack containing 3 single packs of 1 vial of 10 g/200 ml.
Not all pack sizes may be marketed.
Marketing authorization holder:
Kedrion S.p.A.
Loc. Ai Conti, 55051 Castelvecchio Pascoli, Barga (Lucca), Italy
Manufacturer:
Kedrion S.p.A.
55027 Bolognana, Gallicano (Lucca), Italy
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Austria | Ig Vena 50g/l Infusionslösung |
| Germany | Ig Vena 50 g/l Infusionslösung |
| Greece | Ig VENA |
| Italy | IG VENA |
| Poland | Ig VENA |
| Portugal | Ig Vena |
For more information, you should contact your local representative of the marketing authorization holder:
MB&S Medical Business and Science, ul. Chełmska 30/34, 00-725 Warsaw
Phone/fax: 22 851 52 08
Date of last revision of the package leaflet: 11/2020
Information intended for healthcare professionals only:
Instructions for proper use
- Before use, the Ig VENA product should be brought to room temperature or body temperature.
- Before use, the solution should be visually inspected for the presence of particulate matter and discoloration. Solutions that are cloudy or have sediment should not be used.
- Human normal immunoglobulin should be administered intravenously at an initial infusion rate of 0.46 - 0.92 ml/kg/hour (10 - 20 drops per minute) for 20 - 30 minutes. In case of a side effect, the infusion rate should be reduced or the immunoglobulin administration should be discontinued. If it is well tolerated, the infusion rate can be gradually increased to a maximum of 1.85 ml/kg/hour (40 drops per minute).
- In patients with primary immunodeficiency, who tolerate an infusion rate of 0.92 ml/kg/hour, the infusion rate can be gradually increased every 20-30 minutes to 2 ml/kg/hour, 4 ml/kg/hour, and up to a maximum of 6 ml/kg/hour, but only if the patient tolerates the infusion well. Generally, dosing and infusion rate must be adjusted individually to the patient's needs. Depending on the patient's body weight, dosing, and occurrence of side effects, the maximum infusion rate may not be achieved. In case of side effects, the infusion should be discontinued immediately and then resumed at an appropriate rate for the patient.
Special populations
In children and adolescents (0-18 years) and in elderly patients (>64 years), the initial infusion rate should be 0.46 – 0.92 ml/kg/hour (10 – 20 drops per minute) for 20 - 30 minutes. If it is well tolerated, after considering the patient's clinical condition, the infusion rate can be gradually increased to a maximum of 1.85 ml/kg/hour (40 drops per minute).
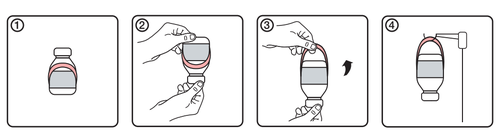
Instructions for using the handle
- 1.Initial appearance of the vial with the handle label
- 2.Invert the vial upside down
- 3.Create the handle by unfolding it from the label
- 4. Hang the vial on the infusion stand
Precautions
Some serious side effects may be related to the infusion rate.
Potential complications can often be avoided by ensuring that:
- patients are not allergic to human normal immunoglobulin, by initial slow administration of the product (infusion rate 0.46 - 0.92 ml/kg/hour);
- patients are closely monitored for side effects during infusion. Especially patients receiving human normal immunoglobulin for the first time, patients who have previously received another IVIg product, or when a long time has passed since the last infusion, should be monitored during the first infusion and for the first hour after the first infusion, to detect potential side effects. Other patients should be observed for at least 20 minutes after infusion.
In all patients, intravenous Ig administration requires:
- adequate hydration before starting the infusion
- monitoring of urine output
- monitoring of serum creatinine levels
- avoidance of concomitant use of loop diuretics. In case of a side effect, the infusion rate should be either reduced or the immunoglobulin administration should be discontinued. The treatment depends on the type and severity of the side effect. In case of anaphylactic shock, you should proceed according to the currently applicable medical standards for anaphylactic shock treatment.
Reaction to infusion
Some side effects (e.g., headache, fever, chills, muscle pain, wheezing, tachycardia, lower back pain, nausea, and vomiting) may be related to the infusion rate.
It is recommended to strictly follow the recommended infusion rate. Patients must be closely monitored and carefully observed during infusion due to the risk of side effects.
Some side effects may occur more frequently:
- in patients who receive human normal immunoglobulin for the first time, or in rare cases, when the previously administered human normal immunoglobulin product is changed to another or when a long time has passed since the last infusion
- in patients with untreated infection or chronic inflammatory condition.
Children and adolescents
There are no special precautions or monitoring requirements for children and adolescents. You should not expect any differences in children and adolescents (from 0 to 18 years).
Thromboembolic events
There is clinical evidence of a link between intravenous Ig administration and thromboembolic events, such as heart attack, stroke, pulmonary embolism, or deep vein thrombosis, which are considered to be related to the relative increase in blood viscosity after intensive immunoglobulin administration in patients at risk. Caution should be exercised when prescribing and administering the product to obese patients and patients at risk of developing thrombotic events (such as elderly patients, those with hypertension, diabetes, or vascular disease, or those with a history of thrombotic events, acquired or congenital coagulation disorders, or prolonged immobilization).
Acute kidney failure
Cases of acute kidney failure have been reported in patients treated with intravenous immunoglobulins. In most cases, risk factors were identified, such as pre-existing kidney failure, diabetes, severe hypovolemia, overweight, or concomitant use of nephrotoxic drugs. In patients at risk of acute kidney failure, intravenous immunoglobulins should be administered at the minimum infusion rate and in the lowest dose possible.
Aseptic meningitis syndrome
During treatment with intravenous immunoglobulins, cases of aseptic meningitis syndrome (AMS) have been reported. The syndrome usually starts within a few hours to 2 days after IVIg administration.
In cerebrospinal fluid examinations, pleocytosis is often found, with several thousand cells per mm^3, mainly granulocytes, and elevated protein levels.
AMS may occur more frequently in association with high-dose IVIg treatment (2 g/kg).
Patients with objective and subjective symptoms should undergo a thorough neurological examination, including cerebrospinal fluid examination, to rule out other causes of meningitis.
Discontinuation of IVIg treatment resulted in remission of AMS within a few days without sequelae.
Hemolytic anemia
Intravenous immunoglobulin products may contain antibodies against blood groups, which can act as hemolysins and induce in vivo coating of red blood cells with immunoglobulin, resulting in a positive direct antiglobulin test (Coombs test) and, rarely, hemolysis.
Patients receiving intravenous immunoglobulin should be monitored for the possibility of hemolysis.
Neutropenia/leukopenia
After IVIg treatment, transient decreases in neutrophil counts and/or episodes of neutropenia have been reported, sometimes severe. This usually occurs within hours or days after IVIg administration and resolves spontaneously within 7 to 14 days.
Acute lung injury related to transfusion (TRALI, Transfusion-related acute lung injury)
In patients receiving IVIg products, cases of acute non-cardiogenic pulmonary edema (acute lung injury related to transfusion - TRALI) have been reported. TRALI is characterized by severe hypoxia, respiratory failure, respiratory distress, cyanosis, fever, and hypotension. TRALI symptoms usually occur within 6 hours after IVIg administration, often within 1-2 hours. Therefore, patients should be monitored; in case of respiratory side effects, IVIg infusion should be discontinued immediately. The occurrence of TRALI can be life-threatening and requires immediate treatment in an intensive care unit.
This medicinal product contains 100 mg of maltose as an excipient in 1 ml. The presence of maltose in the blood may affect the result of glucose testing, giving a falsely elevated reading of blood glucose levels, which may lead to inappropriate administration of insulin, resulting in life-threatening hypoglycemia. Additionally, cases of actual hypoglycemia may remain untreated if the hypoglycemic state is masked by falsely elevated glucose readings. For further information, see section "Blood glucose test".
Dosing
Substitution therapy must be initiated and monitored by a specialist doctor
providing care for immunodeficiency treatment.
Dosing
The dose and dosing regimen depend on the indications. The dose should be individualized for each
patient based on the clinical response. The dose based on body weight may need to be adjusted in patients with underweight or overweight.
The following dosing methods are provided as a guideline.
Substitution therapy in primary immunodeficiency disorders
The dose should be adjusted to achieve an IgG level (measured before the next infusion) of at least 6 g/l or within the normal range for the given population's age. From the start of treatment to the stabilization of the concentration, three to six months are required (stable IgG level). An initial dose of 0.4 - 0.8 g/kg administered once, followed by at least 0.2 g/kg administered every three to four weeks, is recommended. The dose required to achieve a minimum IgG concentration of 6 g/l is 0.2 - 0.8 g/kg/month. After achieving a stable state, the intervals between infusions are 3 - 4 weeks. The immunoglobulin level should be determined and evaluated in relation to the frequency of infections. To reduce the frequency of bacterial infections, it may be necessary to increase the dose to achieve a higher concentration.
Secondary immunodeficiencies
The recommended dose is 0.2 - 0.4 g/kg every three to four weeks.
The minimum IgG level should be determined and evaluated in relation to the frequency of infections. The dose should be adjusted as needed to achieve adequate protection against infections; it may be necessary to increase the dose in patients with persistent infection; a decrease in dose may be considered if the patient has no infection.
Primary immunological thrombocytopenia
Two alternative treatment regimens:
- a dose of 0.8 - 1.0 g/kg on the first day; the dose may be repeated once within 3 days
- 0.4 g/kg per day for two to five days. Treatment may be repeated if the disease recurs.
Guillain-Barré syndrome
0.4 g/kg/day for more than 5 days (possible repetition of dosing in case of recurrence).
Kawasaki disease
A dose of 2.0 g/kg should be administered in a single dose. Patients should receive acetylsalicylic acid concurrently.
Chronic inflammatory demyelinating polyneuropathy (CIDP)
Initial dose: 2 g/kg over 2-5 consecutive days.
Maintenance dose: 1 g/kg over 1 – 2 consecutive days every 3 weeks.
The effectiveness of treatment should be evaluated after each cycle; if no effectiveness is observed after 6 months, treatment should be discontinued.
If therapy is effective, the doctor should decide on long-term treatment, taking into account the patient's reactions and response to maintenance treatment. Dosing and treatment intervals may need to be adjusted based on the individual course of the disease.
Multifocal motor neuropathy (MMN)
Initial dose: 2 g/kg administered over 2 - 5 consecutive days.
Maintenance dose: 1 g/kg every 2 to 4 weeks or 2 g/kg every 4 to 8 weeks.
The effectiveness of treatment should be evaluated after each cycle; if no effectiveness is observed after 6 months, treatment should be discontinued.
If therapy is effective, the doctor should decide on long-term treatment, taking into account the patient's reactions and response to maintenance treatment. Dosing and treatment intervals may need to be adjusted based on the individual course of the disease.
The recommended dosing is presented in the following table:
| Indications | Dose | Infusion frequency |
| Substitution therapy | ||
| Primary immunodeficiency disorders | initial dose: 0.4 - 0.8 g/kg, maintenance dose: 0.2 - 0.8 g/kg | every 3 - 4 weeks |
| Secondary immunodeficiencies | 0.2 - 0.4 g/kg | every 3 - 4 weeks |
| Immunomodulatory therapy | ||
| Primary immunological thrombocytopenia | 0.8 - 1 g/kg or 0.4 g/kg/day | on the first day, possible repetition once within 3 days, for 2 - 5 days |
| Guillain-Barré syndrome | 0.4 g/kg/day | for 5 days |
| Kawasaki disease | 2 g/kg | in a single dose, in combination with acetylsalicylic acid |
| Chronic inflammatory demyelinating polyneuropathy (CIDP) | initial dose: 2 g/kg, maintenance dose: 1 g/kg | in divided doses over 2 - 5 days, every 3 weeks for 1 - 2 days |
| Multifocal motor neuropathy (MMN) | maintenance dose: 1 g/kg or 2 g/kg | every 2 - 4 weeks or every 4 - 8 weeks, over 2 - 5 days |
Use in children and adolescents
Dosing in children and adolescents (0-18 years) does not differ from that in adults, since dosing in individual indications is determined based on body weight and the patient's clinical condition, as described above.
Patients with liver impairment
There are no available data on the need to adjust dosing.
Patients with renal impairment
No dose adjustment is necessary, unless it is clinically justified.
Elderly patients
No dose adjustment is necessary, unless it is clinically justified.
CIDP
Due to the rare occurrence of chronic inflammatory demyelinating polyneuropathy and the consequent small number of patients overall, experience with intravenous immunoglobulin in children with CIDP is limited; only literature data are available.
However, the published data are consistent and all show that IVIg treatment in adults and children is equally effective, as in the case of previously approved indications.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterKedrion S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ig VenaActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Baxalta Belgium Manufacturing S.A.Prescription requiredDosage form: Solution, 100 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Instituto Grifols S.A.Prescription requiredDosage form: Solution, 50 g/l (50 mg/ml)Active substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Biotest Pharma GmbHPrescription required
Alternatives to Ig Vena in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ig Vena in Spain
Alternative to Ig Vena in Ukraine
Online doctors for Ig Vena
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ig Vena – subject to medical assessment and local rules.














