
Gammagard S/d

Ask a doctor about a prescription for Gammagard S/d

How to use Gammagard S/d
GAMMAGARD S/D 50 mg/ml 5 g; 10 g
powder and solvent for solution for infusion
human normal immunoglobulin (IVIg)
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Gammagard S/D and what is it used for
- 2. Important information before using Gammagard S/D
- 3. How to use Gammagard S/D
- 4. Possible side effects
- 5. How to store Gammagard S/D
- 6. Contents of the pack and other information
1. What is Gammagard S/D and what is it used for
Gammagard S/D belongs to a group of medicines called immunoglobulins. These medicines contain human antibodies that are present in human blood. Antibodies help the body fight infections. Medicines like Gammagard S/D are used in cases of antibody deficiency in the blood. Patients with antibody deficiency often get infections. Gammagard S/D can also be used if a patient needs additional antibodies for the treatment of certain inflammatory disorders (autoimmune diseases).
Gammagard S/D is used for the following indications:
Treatment of patients who do not have enough antibodies (replacement therapy).
There are six groups of these patients:
- 1. Patients with congenital absence of antibodies (primary immunodeficiency - PID), such as:
- congenital agammaglobulinemia or hypogammaglobulinemia
- common variable immunodeficiency
- severe combined immunodeficiency
- Wiskott-Aldrich syndrome.
- 2. Patients with blood cancer (chronic lymphocytic leukemia) leading to loss of antibody production and recurrent infections, in whom prophylactic antibiotic treatment has failed.
- 3. Patients with bone marrow cancer (multiple myeloma) leading to loss of antibody production and recurrent infections due to inadequate response to vaccination against certain bacteria (pneumococci).
- 4. Children and adolescents (0-18 years old) with congenital AIDS, who often have infections.
- 5. Premature infants with low birth weight, who are at risk of infection.
- 6. Patients with low antibody levels following a bone marrow transplant from another person.
Treatment of patients with certain inflammatory disorders (immunomodulation). There are three groups of these patients:
- 1. Patients with a low platelet count (with primary immune thrombocytopenia) and a high risk of bleeding or planned surgery in the near future.
- 2. Patients with a disease associated with multiple nerve inflammations throughout the body (Guillain-Barré syndrome).
- 3. Patients with a disease that causes multiple organ inflammation (Kawasaki disease).
2. Important information before using Gammagard S/D
When not to use Gammagard S/D
DO NOT USE Gammagard S/D
- if you are allergic to immunoglobulins or any of the other ingredients of this medicine listed in section 6.
- in case of immunoglobulin A (IgA) deficiency. The patient's blood may contain antibodies against immunoglobulin A. However, Gammagard S/D contains only very small amounts of immunoglobulin A (no more than 3 micrograms/ml in a 5% solution).
Warnings and precautions
Before starting treatment, discuss it with your doctor, pharmacist, or nurse.
For how long during infusion is close monitoring of the patient required
- During Gammagard S/D infusion, the patient will be under close observation to ensure that no abnormal reaction occurs. The doctor will ensure that the rate of administration of Gammagard S/D is appropriate,
- if Gammagard S/D is administered at a high rate,
- if the patient has a disease with low antibody levels in the blood (hypo- or agammaglobulinemia),
- in patients who have not received this medicine before,
- if there has been a long interval (e.g., several weeks) since the last administration of the medicine. In such cases, during administration and for one hour after completion of the infusion, the patient should be subject to close observation.
- If the patient has recently received Gammagard S/D, they should be monitored during infusion and for at least 20 minutes after administration of the medicine.
When it may be necessary to reduce the rate or interrupt the infusion
In very rare cases, the patient's body may show sensitivity to medicines containing antibodies. This can occur especially in cases of immunoglobulin A deficiency. In these rare cases, allergic reactions, such as a sudden drop in blood pressure or shock, may occur, even if the patient has been treated with antibody-containing medicines before.
- If any of the following side effects occur, inform your doctor or nurse immediately:
- Sudden breathing difficulties (wheezing), difficulty breathing, or chest tightness
- Headache
- Fever
- Swelling of the eyelids, face, lips, or blood vessels
- Hives or itchy red spots
- Itching all over the body. Depending on the doctor's decision, the rate of administration of the medicine will be reduced or the infusion will be interrupted.
Special patient groups
- The doctor will exercise special caution in patients with overweight, elderly, diabetic, or immobilized patients. The doctor will closely monitor patients:
- suffering from hypertension
- with decreased blood volume (hypovolemia)
- with increased blood viscosity or vascular problems (vascular diseases).
- In the case of such conditions, immunoglobulins may increase the risk of heart attack, stroke, pulmonary embolism, or deep vein thrombosis, although only in very rare cases. If the patient has diabetes, they should inform their doctor. Gammagard S/D does not contain sucrose or maltose. The 5% solution of Gammagard S/D contains approximately 20 mg/ml of glucose, which may affect the patient's blood sugar level.
- The doctor will also exercise special caution
- if the patient has kidney problems or is taking medicines that may be harmful to the kidneys (nephrotoxic medicines), as there is a very rare risk of acute kidney failure. Inform your doctor about any kidney problems.
The protein content may increase, causing an increase in blood viscosity.
Information about the raw material from which Gammagard S/D is made
Gammagard S/D is made from human plasma (the liquid part of the blood). In the case of medicines made from human blood or plasma, numerous measures are taken to prevent the transmission of infections. These include careful selection of donors to exclude individuals at risk of transmitting infections, and testing of individual donations and plasma pools for the presence of viruses/infections. Manufacturers of such medicines also include virus inactivation or removal steps in the blood and plasma processing process. Despite this, it is not possible to completely rule out the possibility of transmitting infectious agents when administering medicines made from human blood or plasma. This includes unknown or newly discovered viruses and other types of infections.
Actions taken during the manufacture of Gammagard S/D are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, as well as against non-enveloped viruses such as hepatitis A virus and parvovirus B19. Gammagard S/D also contains specific antibodies that may prevent hepatitis A virus or parvovirus B19 infections.
Gammagard S/D and other medicines
- Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take or vaccinations you have received in the last six weeks.
- Infusion of immunoglobulins such as Gammagard S/D may weaken the effectiveness of certain live virus vaccines, such as measles, rubella, mumps, and chickenpox vaccines. Vaccination with live, attenuated virus vaccines should be performed only after 3 months from the last administration of Gammagard S/D. In the case of measles vaccine, it may be necessary to extend this period to one year.
Effect on laboratory tests
Gammagard S/D contains a wide range of diverse antibodies, some of which may affect laboratory test results. When undergoing blood tests, inform the person taking the blood or your doctor that you are taking Gammagard S/D.
Administration of human immunoglobulins may cause false-positive results for beta-D-glucan detection in serum for the diagnosis of fungal infections; this condition may persist for a longer period after infusion of the medicine.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine. The doctor will decide whether Gammagard S/D can be used during pregnancy or breastfeeding.
- No clinical studies have been conducted with the use of Gammagard S/D in pregnant or breastfeeding women. Long-term clinical experience with the use of antibody-containing medicines suggests that they do not have a harmful effect on the course of pregnancy, the fetus, or the newborn.
- During breastfeeding, antibodies contained in Gammagard S/D may appear in breast milk. This way, the child may be protected against certain infections.
Driving and using machines
Patients may experience symptoms (e.g., nausea or dizziness) while taking Gammagard S/D, which may affect their ability to drive vehicles and operate machines.
If this occurs, wait until these symptoms have resolved.
Important information about some ingredients of Gammagard S/D
The medicine contains about 2.0 g of glucose per vial of 5 g and about 4.0 g of glucose per vial of 10 g. This should be taken into account in patients with diabetes.
The medicine contains about 334 mg of sodium (the main component of common salt) in each vial of 5 g and about 668 mg of sodium in each vial of 10 g. This corresponds to approximately 17% and approximately 34% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use Gammagard S/D
Gammagard S/D is intended for intravenous administration (intravenous infusion). The medicine is administered by a doctor or nurse. The dosing regimen and frequency of infusions vary depending on the patient's clinical condition and body weight.
Initially, Gammagard S/D will be administered at a low rate. Depending on how the infusion is tolerated, the doctor may gradually increase the infusion rate.
Use in children
The same indications, dosing, and frequency of infusions apply to adults and children (0-18 years old).
Use of a higher than recommended dose of Gammagard S/D
Overdose of the medicine may lead to excessive blood thickening (excessive blood viscosity). The thicker the blood, the harder it moves through the body's vessels. As a result, less oxygen is delivered to important organs such as the brain, lungs, etc. This may occur especially in patients at risk, i.e., elderly patients or patients with heart or kidney problems. Ensure that the patient has taken the appropriate amount of fluids and is not dehydrated and inform your doctor about health problems.
4. Possible side effects
Like all medicines, Gammagard S/D can cause side effects, although not everybody gets them.
However, possible side effects can be limited by reducing the infusion rate.
The following side effects may occur after treatment with immunoglobulins (medicines such as Gammagard S/D):
- Frequent or not very frequent side effects (which occur in less than 1 in 10 patients but more than 1 in 1,000 patients) include: chills, headaches, fever, vomiting, allergic reactions, nausea (feeling sick), joint pain, low blood pressure, and moderate back pain.
- Rare side effects, which occur in less than 1 in 1,000 patients, include:
- cases of sudden drop in blood pressure
- cases of symptoms resembling rash (transient skin reactions)
- Very rare side effects, which occur in less than 1 in 10,000 patients or whose frequency cannot be estimated from the available data, include:
- single cases of allergic reactions (anaphylactic shock), also in patients who have not shown any reactions to previous administrations,
- cases of transient meningitis (aseptic meningitis),
- single cases of transient red blood cell destruction (reversible hemolytic anemia/hemolysis),
- transient increases in liver enzymes (liver transaminases), increased creatinine levels in the blood, and kidney failure,
- blood clots in veins (thromboembolic reactions), which can lead to heart attack, pulmonary embolism, and deep vein thrombosis.
The following is a list of side effects that have been reported during clinical trials with Gammagard S/D and after the medicine has been on the market:
- Very rare side effects, which occur in less than 1 in 10,000 patients or whose frequency cannot be estimated from the available data: none
- Frequent side effects (which occur in less than 1 in 10 patients) include: headache, facial flushing, nausea, vomiting, fatigue, chills, fever.
- Uncommon side effects (which occur in less than 1 in 100 patients): flu, nervousness, agitation, abnormal sleepiness, blurred vision, irregular heartbeat, shortness of breath, nosebleeds, diarrhea, abdominal pain, liver inflammation (non-infectious), skin redness, rash, skin inflammation, allergic swelling of deep skin layers, joint and muscle pain, kidney failure, general weakness, swelling of body tissues, reactions at the injection site and infusion, chills, positive Coombs test result.
- Side effects of unknown frequency (whose frequency cannot be estimated from the available data) include: aseptic meningitis, destruction of red blood cells, decrease in red blood cell count, decrease in platelet count, lymph node enlargement, all degrees of allergic reactions including anaphylactic shock, anxiety, dizziness, abnormal skin changes, tremors, seizures, cerebral hemorrhage, migraine, loss of consciousness, photophobia, vision disturbances, eye pain, closure of the main vein in the eye, heart attack, bluish discoloration of the skin, increased heart rate, decreased heart rate, high blood pressure, pallor, low blood pressure, vein inflammation, closure of blood vessels, cough, throat tightness, decreased oxygen levels in the blood, hyperventilation, wheezing, bronchospasm, closure of blood vessels in the lungs, fluid in the lungs, digestive disorders, abdominal pain, liver inflammation (non-infectious), skin redness, rash, skin inflammation, allergic swelling of deep skin layers, joint and muscle pain, kidney failure, general weakness, swelling of body tissues, reactions at the injection site and infusion, chills, positive Coombs test result.
If any of the side effects get worse or if you experience any side effects not listed in this leaflet, inform your doctor, pharmacist, or nurse.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181 C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Gammagard S/D
- Store out of sight and reach of children.
- Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of the month.
- Do not use this medicine if you notice any solid particles or a change in color.
- Store at a temperature below 25°C.
- Do not freeze.
- Store the vials in the outer carton to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Gammagard S/D contains
- The active substance of Gammagard S/D is human normal immunoglobulin. Gammagard S/D is reconstituted in water for injections to obtain a 5% (50 mg/ml) protein solution. At least 90% of the protein is immunoglobulin G (IgG).
Gammagard S/D can be reconstituted in water for injections to obtain a 10% (100 mg/ml) protein solution.
- The other ingredients are: human albumin, glycine, sodium chloride, and glucose monohydrate.
What Gammagard S/D looks like and contents of the pack
Gammagard S/D is a lyophilized, white or pale yellow powder, without visible foreign particles.
Gammagard S/D is available in the following pack sizes: 5 g and 10 g.
Gammagard S/D; 5 g and 10 g: Each pack contains
- a vial of powder of 5 g or 10 g
- 96 ml or 192 ml of water for injections
- a transfer device
- a set for administration equipped with a filter.
Marketing authorization holder
Takeda Pharma Sp. z o.o.
Prosta Street 68
00-838 Warsaw
Manufacturer
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
7860 Lessines, Belgium
Date of last revision of the leaflet:
Information intended for healthcare professionals only:
Special precautions for storage
If the solution is prepared under aseptic conditions outside a laminar flow cabinet, administration should be started as soon as possible, but no later than 2 hours after preparation of the solution. If the solution is prepared under aseptic conditions in a laminar flow cabinet, the ready-to-use solution can be stored in the refrigerator (2°C - 8°C) for up to 24 hours. If these conditions are not met, the sterility of the reconstituted product may not be maintained. Vials with partially used solution should be discarded according to the applicable procedures.
Reconstitution – follow aseptic technique:
- A. 5% solution:
- 1. Bring Gammagard S/D and water for injections (solvent) to room temperature. This temperature should be maintained until the powder is completely dissolved.
- 2. Remove the cap from the vial of powder and the vial of solvent to expose the central part of the rubber stoppers.
- 3. Disinfect the surfaces of the rubber stoppers with a bactericidal solution.
- 4. Remove the protective cap from one end of the transfer device.
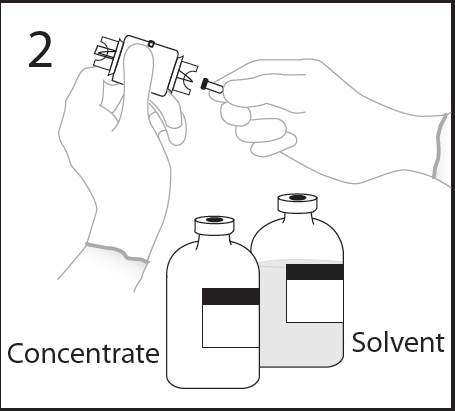
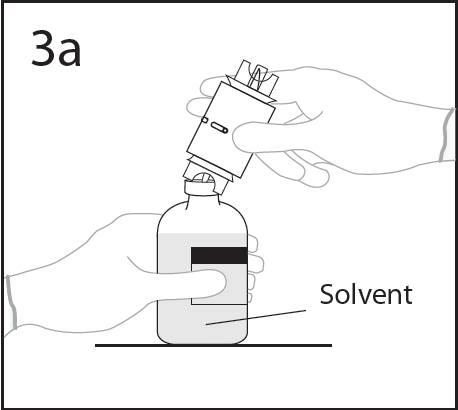
- 5. Place the vial of solvent on a flat surface, holding the vial to prevent it from slipping, and pierce the centerof the rubber stopper of the vial of solvent perpendicularlywith the spike of the transfer device.
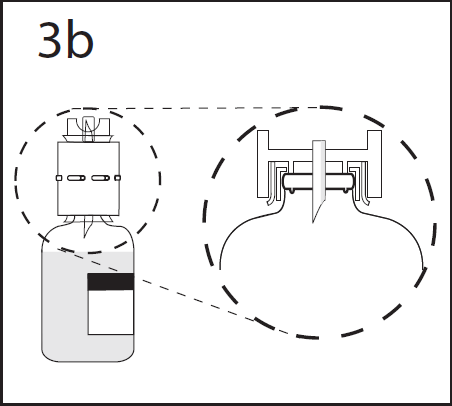
- 6. Press the transfer device firmly to ensure that the collar is fully seated in the device.
WARNING: Insertion of the spike beyond the center of the stopper may cause the stopper to be dislodged.
- 7. Remove the protective cap from the other end of the transfer device. Hold the transfer device to prevent it from slipping.
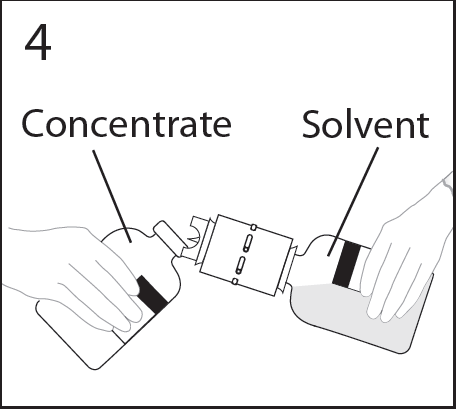
- 8. Hold the vial firmly and at an angle of about 45 degrees. Tilt the vial of solvent and attached transfer device to the vial of powder (at an angle of about 45 degrees) and firmly insert the transfer device into the centerof the rubber stopper of the vial of powder.
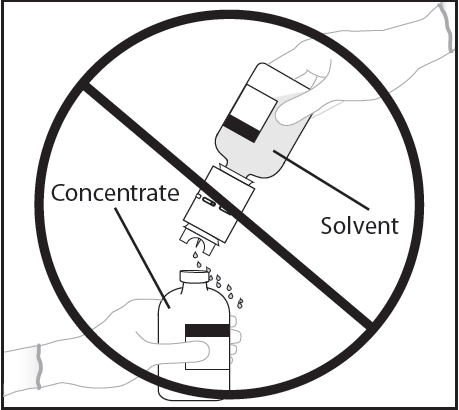
WARNING: Quickly invert the vial of solvent and attached transfer device to the vial of powder to avoid spilling the solvent.
WARNING: Insertion of the spike beyond the center of the stopper may cause the stopper to be dislodged and loss of vacuum.
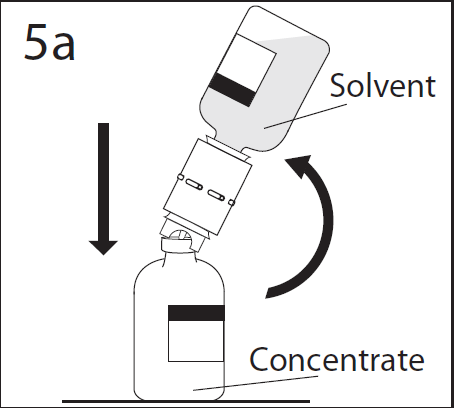
- 9. The solvent will rapidly flow into the vial of powder. After completion of the transfer, separate the empty vial of solvent and transfer device from the vial of powder. Discard the transfer device after single use.
- 10. Moisten the dry powder thoroughly by tilting or inverting and gently rotating the vial. Do not shake. Do not foam the solution.
- 11. Repeat gentle mixing by rotating the vial contents until any undissolved product is no longer visible.
- B. 10% solution: Follow the same procedure as described above in points 1-3 of section A.
- 4. Reconstitution of the powder using the appropriate volume of solvent with a sterile needle for subcutaneous injection and a syringe. To obtain a 10% solution, 48 ml of solvent is needed for 5.0 g of powder, and 96 ml of solvent is needed for 10 g of powder. Maintaining aseptic technique, draw the required volume of solvent into a sterile syringe with a needle for subcutaneous injection. Discard the filled syringe.
- 5. Using the remaining solvent in the vial, perform steps 4-11 as previously described in section A.
Administration – follow aseptic technique: 5 g; 10 g set
Administer the solution according to the instructions for use provided with the administration set, which is included in each package. If a different administration set is used, ensure that it contains a similar filter.
Instructions for preparing the medicine for use and disposing of its remnants
- The powder should dissolve completely within 30 minutes.
- The medicine should be warmed to room temperature or body temperature before use.
- The solution should be clear or slightly opalescent and colorless or pale yellow. A cloudy or precipitated solution is not suitable for use. The reconstituted solution should be visually inspected for particulate matter and discoloration prior to administration.
- Unused medicine or solution remnants should be disposed of according to the applicable procedure.
- Discard the transfer device after single use.
Method of administration
For intravenous use.
If possible, it is recommended to administer 10% gammaglobulin solutions into the elbow veins. This may reduce the likelihood of discomfort experienced by the patient at the infusion site.
Gammagard S/D in the form of a 5% solution (50 mg/ml) should be administered intravenously at an initial rate of 0.5 ml/kg body weight/hour. In general, it is recommended that patients starting treatment with Gammagard S/D or switching from one manufacturer's immunoglobulin to another start with the lowest rate and then increase it to the maximum rate if the patients tolerate several infusions administered at an average rate. If the infusion is well tolerated, the rate of administration can be gradually increased to a maximum of 4.0 ml/kg body weight/hour. For patients who tolerate the 5% solution of Gammagard S/D at a rate of 4 ml/kg body weight/hour, administration of the 10% solution can be started at an initial rate of 0.5 ml/kg body weight/hour. If no adverse reactions occur, the infusion rate can be gradually increased to a maximum of 8 ml/kg body weight/hour.
Special warnings
- In case of any infusion-related adverse reactions, reduce the rate of administration or interrupt the infusion.
- It is recommended that the name and batch number of Gammagard S/D be recorded after each administration.
Incompatibilities
Gammagard S/D must not be mixed with other medicines.
It is recommended that Gammagard S/D be administered separately when the patient is taking other medicines.
Dosing recommendations
| Indication | Dose | Frequency of administration |
| Replacement therapy in primary immunodeficiency syndromes Replacement therapy in secondary immunodeficiency syndromes Congenital AIDS Premature infants with low birth weight (neonates up to 7 days of life) Hypogammaglobulinemia (<4 g l) in patients after allogeneic hematopoietic stem cell transplantation
| initial dose: 0.4-0.8 g/kg body weight, then 0.2-0.8 g/kg body weight, 0.2-0.4 g/kg body weight, 0.2-0.4 g/kg body weight, 0.5 g/kg body weight, 0.2-0.4 g/kg body weight | every 3-4 weeks to achieve an IgG level of at least 5.0-6.0 g/l, every 3-4 weeks to achieve an IgG level of at least 5.0-6.0 g/l, every 3-4 weeks, 2 infusions at an interval of 1 week, and then 5 subsequent infusions every 14 days or until hospital discharge, every 3-4 weeks to achieve an IgG level of at least 5.0 g/l, once a week, starting from 7 days before and continuing for 3 months after transplantation, once a month until normal antibody levels are restored |
| Immunomodulation: Primary immune thrombocytopenia (ITP) Guillain-Barré syndrome Kawasaki disease | 0.8-1.0 g/kg body weight or 0.4 g/kg body weight/day, 0.4 g/kg body weight/day, 1.6-2.0 g/kg body weight or 2.0 g/kg body weight | on the first day, with the possibility of repetition once within 3 days, for 2-5 days, for 5 days, in several divided doses over 2-5 days, in combination with acetylsalicylic acid, as a single dose in combination with acetylsalicylic acid |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBaxalta Belgium Manufacturing S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Gammagard S/dDosage form: Solution, 100 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Instituto Grifols S.A.Prescription requiredDosage form: Solution, 50 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Kedrion S.p.A.Prescription not requiredDosage form: Solution, 50 g/l (50 mg/ml)Active substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Biotest Pharma GmbHPrescription required
Alternatives to Gammagard S/d in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Gammagard S/d in Spain
Alternative to Gammagard S/d in Ukraine
Online doctors for Gammagard S/d
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Gammagard S/d – subject to medical assessment and local rules.














