

Gamunex 10%

Ask a doctor about a prescription for Gamunex 10%

How to use Gamunex 10%
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: information for the user
Gamunex 10%
100 mg/ml Solution for infusion
Human normal immunoglobulin (IVIg)
Please read this leaflet carefully before using this medicine, as it contains important information for you.
Important information for patients.
- You should keep this leaflet, so you can read it again if you need to.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Contents of the leaflet:
- 1. What is Gamunex 10%and what is it used for
- 2. Important information before using Gamunex 10%
- 3. How to use Gamunex 10%
- 4. Possible side effects
- 5. How to store Gamunex 10%
- 6. Contents of the pack and other information
1.
What is Gamunex 10%and what is it used for
What is Gamunex 10%
The medicinal product Gamunex10%contains human normal immunoglobulins (antibodies), i.e.
highly purified protein obtained from human plasma (a blood component obtained from donors). This medicine
belongs to a group of medicines called intravenous immunoglobulins. They are used to treat diseases in which
the body's defense system does not work properly.
Use of Gamunex 10%:
Treatment of adults, children and adolescents (0-18 years) who do not have enough antibodies (replacement therapy), such as:
- patients with primary immunodeficiency syndromes (PID), i.e. congenital lack of antibodies;
- patients with acquired immunodeficiency (SID) with severe or recurrent infections, for whom antimicrobial therapy is ineffective and who have confirmed failure to produce specific antibodiesor IgG serum concentration <4 g l.< li>
Prevention of measles before/after exposure in susceptible adults, children and adolescents (0-18 years), for whom active vaccination is contraindicated or not recommended and who are at risk of contracting the disease in the future or after exposure to measles.
Treatment of adults, children and adolescents (0-18 years) with certain autoimmune diseases (immunomodulation). These are five groups:
- Primary immunological thrombocytopenia (ITP); a disease in which the number of platelets in the blood is significantly too low. Platelets are an essential part of the blood clotting process and a decrease in their number can cause unwanted bleeding and bruising. The product is also used in patients with a high risk of bleeding or to correct platelet count before surgery.
- Guillain-Barré syndrome, in which the immune system damages the nerves and disrupts their normal function.
- Kawasaki disease (in combination with acetylsalicylic acid) - a childhood disease in which the blood vessels (arteries) in the body become inflamed.
- Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) - a rare, progressive disease causing weakness, numbness and pain in the limbs, as well as fatigue.
- Multifocal motor neuropathy (MMN) - a rare disease resulting in slow, progressive weakness of the limbs without loss of sensation.
Treatment of patients over 18 years of age:
- Severe exacerbations of myasthenia. Myasthenia is a disease that causes excessive muscle fatigue. Exacerbations of myasthenia cause weakness of swallowing, speech and breathing disorders.
2.
Important information before using Gamunex 10%
When not to use Gamunex 10%:
- if you are allergic to human normal immunoglobulins or any of the other ingredients of this medicine (listed in section 6),
- if you have IgA deficiency in your blood and produce antibodies against IgA.
Warnings and precautions
Before starting treatment with Gamunex 10%, discuss it with your doctor, pharmacist or nurse.
Infusion-related reactions and hypersensitivity
Some side effects may be related to the infusion rate. Therefore, the recommended infusion rate should be followed (see " Information intended exclusively for
healthcare professionals" at the end of this leaflet )).
Some side effects may occur more frequently:
with high infusion rates,
in patients with complete lack of gammaglobulin or low gammaglobulin levels (agammaglobulinemia or hypogammaglobulinemia) with or without IgA deficiency,
in patients who are administered human immunoglobulin for the first time or, in rare cases, in patients who have changed their previously used immunoglobulin preparation to another and in patients who have been given immunoglobulin after a long break.
It is often possible to avoid potential complications by ensuring that:
patients are not hypersensitive to human normal immunoglobulin – by starting Gamunex 10%slowly.
patients are closely monitored during infusion, paying attention to any signs of adverse reactions. Particular attention is required for patients who are administered human immunoglobulin for the first time, who have changed their previously used immunoglobulin preparation to another and patients who are given immunoglobulin after a long break. This group of patients should be monitored during the first infusion and for the first hour after infusion to notice any signs of possible side effects.
If side effects occur, the infusion rate should be reduced or the infusion stopped until the symptoms disappear. If the symptoms persist despite stopping the infusion, appropriate treatment should be applied. If anaphylactic shock (with a sudden drop in blood pressure) occurs, the administration of the medicine should be stopped immediately and treatment should be started in accordance with current guidelines for anaphylactic shock.
Patients with kidney problems and other risk factors
There have been reports of kidney problems and acute kidney failure associated with intravenous immunoglobulin administration. Patients with pre-existing kidney failure (kidney problems), diabetes (high blood sugar), hypovolemia (reduced blood volume), overweight; patients treated with medications that may have a harmful effect on the kidneys and patients over 65 years of age are particularly at risk. The following recommendations should be followed:
before starting treatment, ensure adequate hydration
monitor diuresis and serum creatinine levels (allowing kidney function to be assessed) and
avoid concomitant use of medications that increase urine production (loop diuretics).
In these patients, the immunoglobulin preparation should be administered during infusion at the minimum rate, at the minimum effective concentration. If kidney problems occur, the doctor will consider stopping immunoglobulin treatment.
Hemolysis (abnormal breakdown of red blood cells)
It is widely known that immunoglobulins increase the risk of red blood cell breakdown (hemolysis) in both adults and children. The increased risk of red blood cell breakdown (hemolysis) may occur if high doses of IVIg are administered within one day or within several days to patients with blood group A, B or AB and/or when there is an inflammatory condition in the body.
According to existing experience, immunoglobulin preparations do not transmit hepatitis A virus or parvovirus B19. It is assumed that the presence of antibodies plays a significant protective role against viral infections.
The medicine contains less than 1 mmol of sodium (23 mg) per single dose (up to 2 g/kg), i.e. the medicine is considered "sodium-free".
It is strongly recommended that when administering this medicine, its name and batch number should be recorded, so that the batch of the medicine administered to the patient can be identified.
Gamunex 10%and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
During treatment with Gamunex 10%, avoid concomitant use of medications that increase urine production (loop diuretics) .
Effect on vaccinations: Gamunex 10%may reduce the effectiveness of some vaccines (vaccines containing live attenuated viruses). In the case of vaccinations against measles, mumps and rubella, vaccinations can be started only after 3 months from the administration of this product. In the case of measles vaccination, this period is up to 1 year.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or plan to have a child, consult your doctor or pharmacist before using this medicine.
Driving and using machines
As dizziness and other reactions may occur, the medicine may affect your ability to drive and use machines. In this case, wait until these reactions have passed before driving vehicles and operating machines.
3.
How to use Gamunex 10%
The medicine Gamunex10%is injected into a vein by a doctor.
The dose, which depends on the patient's weight and the disease they are suffering from, is determined by the doctor (see also the information intended for healthcare professionals at the end of this leaflet).
At the beginning of the infusion, the medicine Gamunex 10%is administered at a slow rate. If the medicine is well tolerated, the doctor may increase the infusion rate.
Stopping Gamunex 10%treatment
After stopping treatment with this medicine, the patient's condition may worsen. Before deciding to stop treatment with this medicine, consult your attending doctor.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicinecan cause side effects, although not everybody gets them.
The symptoms listed below have occurred in rare or single cases after administration of immunoglobulin-containing medicines. If the following symptoms occur during or after the end of the infusion,
seek medical help immediately:
- sudden drop in blood pressure, and in some cases anaphylactic shock (whose symptoms include, among others, rash, low blood pressure, rapid or irregular heartbeat, wheezing, coughing, and difficulty breathing), even if there were no symptoms of an allergic reaction after previous doses of the medicine;
- transient non-infectious meningitis (whose symptoms are headache, feeling of anxiety or intolerance to light, stiffness of the neck);
- transient reduction in red blood cell count (reversible hemolytic anemia/hemolysis);
- transient skin reactions;
- increase in serum creatinine levels (a test that assesses kidney function) and/or acute kidney failure (whose symptoms are pain in the lumbar region, fatigue, decreased urine output);
- thromboembolic events such as myocardial infarction (feeling of pressure in the chest giving a feeling of excessive heart action), stroke (weakness of facial muscles, arms or legs, speech disorders or difficulty understanding speech), pulmonary embolism (feeling of shortness of breath, chest pain and fatigue), deep vein thrombosis (pain and swelling of the limbs);
- cases of acute post-transfusion pulmonary edema (TRALI) causing hypoxia (lack of oxygen), dyspnea (difficulty breathing), rapid breathing, cyanosis, fever, and low blood pressure.
In clinical trials of Gamunex 10%, the following side effects were observed:
The following side effects occurred frequently (in up to 1 in 10 infusions):
- headache
- fever
The following side effects occurred uncommonly (in up to 1 in 100 infusions):
- dizziness
- urticaria (redness, itching of the skin)
- pruritus (itching)
- rash
- nausea
- vomiting
- increased blood pressure
- pharyngitis
- cough
- nasal congestion
- wheezing
- arthralgia
- back pain
- flu-like symptoms
- fatigue
- chills
- asthenia (weakness)
- myalgia
The following side effects occurred rarely (in up to 1 in 1000 infusions):
- hemolytic anemia (breakdown of red blood cells)
- shortness of breath
- sinusitis
- exfoliative dermatitis
- anxiety
- decreased hemoglobin levels
- gastrointestinal disorders
- bruising
- flushing
- muscle stiffness
- palmar erythema (redness of the palms)
- aphonia (loss of voice)
- decreased white blood cell count
- skin or contact dermatitis
- abdominal pain
- diarrhea
- decreased blood pressure
- neck pain
- musculoskeletal pain
- chest pain
- malaise
- infusion site reactions
- urethritis (painful urination or difficulty urinating)
- upper respiratory tract viral infections (diseases caused by acute upper respiratory tract infections, including the nose, sinuses and throat)
- lymphocytosis (increased number of a certain type of white blood cell)
- hypersensitivity (allergic reactions)
- photosensitivity
- hypertensive crisis (sudden increase in blood pressure)
- congestion
- hemoglobinuria (presence of oxygen-transporting protein in urine at an abnormally high concentration)
- increased blood pressure
- presence of free hemoglobin (hemoglobin circulating outside red blood cells)
- increased ESR (increased erythrocyte sedimentation rate)
What to do if side effects occur?
If side effects occur, the infusion rate should be reduced or the infusion stopped until the symptoms disappear. If the symptoms persist despite stopping the infusion, appropriate treatment should be applied.
In the case of a severe hypersensitivity reaction with a drop in blood pressure and shortness of breath, severe generalized allergic reaction (including anaphylactic shock), the treatment with this medicine should be stopped immediately and appropriate treatment should be started.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: + 48 22 49 21 301, Fax: + 48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5.
How to store Gamunex 10%
This medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and on the vial. The shelf life is 3 years.
Store in a refrigerator (2 – 8 °C). Do not freeze. Store the vial in the outer packaging.
The medicine can be stored in the original outer packaging for up to 6 months at room temperature (not above 25°C). The shelf life expires after 6 months of storage at room temperature. A new expiry date must be written on the outer packaging. The new expiry date must not be later than the expiry date printed on the packaging. Re-cooling is not allowed.
After opening the ampoule, the medicine should be used immediately. Any unused residue should be discarded. Due to the risk of microbiological contamination, re-storage of the preparation, even in the refrigerator, is not allowed.
6. Contents of the pack and other information
What Gamunex 10%contains:
The active substance of the medicine is human normal immunoglobulin (IVIg).
1 ml of this medicine contains 100 mg of protein, including at least 98% IgG in water for injection.
One vial of 10 ml contains: 1 g of human normal immunoglobulin
One vial of 50 ml contains: 5 g of human normal immunoglobulin
One vial of 100 ml contains: 10 g of human normal immunoglobulin
One vial of 200 ml contains: 20 g of human normal immunoglobulin
One vial of 400 ml contains: 40 g of human normal immunoglobulin.
The percentage of IgG subclasses is approximately: 62.8% (IgG1), 29.7% (IgG2), 4.8% (IgG3), 2.7% (IgG4).
Maximum IgA content: 84 micrograms/ml;
The other ingredient is: glycine and water for injection.
What Gamunex 10%looks like and what the pack contains:
Gamunex 10%is a solution for infusion. The solution is clear to slightly opalescent, colorless or pale yellow.
Gamunex 10%is available in packs of: 10 ml, 50 ml, 100 ml, 200 ml and 400 ml. The pack contains a glass vial with a stopper (made of chlorobutyl rubber), a label with a hanging device and a patient information leaflet.
Marketing authorization holder and manufacturer:
Marketing authorization holder:
Grifols Deutschland GmbH
Colmarer Straße 22
60528 Frankfurt
Germany
Tel.: +49 69/660 593 100
Manufacturer:
Instituto Grifols, S.A.
Can Guasc, 2 – Parets del Vallès
08150 Barcelona
Spain.
This medicinal product is authorized in the Member States of the European Economic Area and the United Kingdom (Northern Ireland) under the following names:
Austria, Belgium, Cyprus, Netherlands, Ireland, Luxembourg, Germany, Poland, Portugal, United Kingdom (Northern Ireland): Gamunex 10% 100 mg/ml
Czech Republic, Denmark, Finland, France, Spain, Norway, Slovakia, Sweden, Hungary, Italy: Gamunex
100 mg/ml
Greece: Gaminex 10% 100 mg/ml
Date of last revision of the leaflet:09/2022
-------------------------------------------------------------------------------------------------------------------------
Information intended exclusively for healthcare professionals
Use only completely clear or slightly opalescent and colorless or pale yellow solutions that do not contain particles.
Do not shake. Before administration, bring Gamunex 10%to room temperature or body temperature (if possible in a water bath at a temperature not exceeding 37°C).
The vials are supplied with a label with an integrated hanging device (Fig. 1). After connecting the infusion set (Fig. 2), bend the loop-shaped part of the label (Fig. 3). Pull firmly and
decisivelyon the loop, holding it firmly with your fingers where it connects to the rest of the label (Fig. 4). Hang the vial on the stand by the created loop (Fig. 5).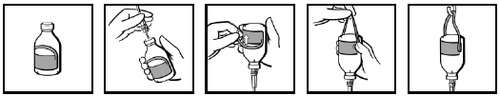
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Dosage and administration
Dose and dosing schedule depend on the indication.
It may be necessary to adjust the doses individually for each patient, depending on the clinical response. Dosing based on body weight may need to be adjusted in patients who are underweight or overweight. The following dosing schedules are provided as guidelines.
The recommended dosing is summarized in the following table:
| Indication | Dose | Infusion frequency |
| Replacement therapy | ||
| Primary immunodeficiency syndromes | Initial dose: 0.4–0.8 g/kg Initial dose: 0.2–0.8 g/kg | every 3–4 weeks |
| Secondary immunodeficiency | 0.2–0.4 g/kg | every 3–4 weeks |
| Prophylaxis of measles before/after exposure | ||
| Post-exposure prophylaxis in susceptible patients | 0.4 g/kg | As soon as possible and within 6 days of exposure, with the possibility of repeating once after 2 weeks to maintain antibody levels against measles at > 240 mIU/ml |
| Post-exposure prophylaxis in patients with PID/SID | 0.4 g/kg | An additional dose to maintenance therapy within 6 days of exposure. |
| Pre-exposure prophylaxis in patients with PID/SID | 0.53 g/kg | If the patient receives a maintenance dose of <0.53 g/kg every 3–4 weeks, the dose should be increased to at least 0.53 g/kg |
| Immunomodulatory treatment: | ||
| Primary immunological thrombocytopenia | 0.8–1 g/kg or 0.4 g/kg/day | on day 1, with the possibility of repeating once within 3 days for 2–5 days |
| Guillain-Barré syndrome | 0.4 g/kg/day | for 5 days |
| Kawasaki disease | 2 g/kg | in a single dose in combination with acetylsalicylic acid |
| Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) | Initial dose: 2 g/kg Maintenance dose: 1 g/kg | in divided doses over 2–5 days every 3 weeks for 1–2 days in divided doses |
| Multifocal motor neuropathy (MMN) | Initial dose: 2 g/kg Maintenance dose: 1 g/kg or 2 g/kg | in divided doses over 2–5 consecutive days every 2–4 weeks or every 4–8 weeks in divided doses over 2–5 days |
| Indication | Dose | Infusion frequency |
| Severe exacerbations of myasthenia | 2 g/kg | In divided doses over 2 days (1 g/kg per day) |
Administration route
For intravenous use.
Human immunoglobulin should be administered intravenously at an initial infusion rate of 0.6–1.2 ml/kg/hour for half an hour. If adverse reactions occur, the infusion rate should be reduced or the infusion stopped. If the infusion is well tolerated, the infusion rate can be gradually increased to a maximum rate of 4.8–8.4 ml/kg/hour.
Children and adolescents
Dosing in children and adolescents (0–18 years) does not differ from dosing in adults, as the dosing for each indication is given per kilogram of body weight and adjusted according to the clinical outcome in the above-mentioned diseases.
Do not mix Gamunex 10%with other infusion solutions or other medicines.
If dilution of the medicine is necessary before administration, use glucose solution 50 mg/ml for this purpose.
Do not dilute with saline solutions.
Avoid concomitant administration of Gamunex 10%and heparinthrough the same infusion device.
Infusion lines for Gamunex10% can be flushed with glucose solution 50 mg/ml or sodium chloride (9 mg/ml) and must notbe flushed with heparin solutions.
Systems for administering Gamunex 10%can be flushed with glucose solution 50 mg/ml or sodium chloride (9 mg/ml) and must notbe flushed with heparin solutions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterInstituto Grifols, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Gamunex 10%Active substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Baxalta Belgium Manufacturing S.A.Prescription requiredDosage form: Solution, 50 mg/mlActive substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Kedrion S.p.A.Prescription not requiredDosage form: Solution, 50 g/l (50 mg/ml)Active substance: immunoglobulins, normal human, for intravascular adm.Manufacturer: Biotest Pharma GmbHPrescription required
Alternatives to Gamunex 10% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Gamunex 10% in Spain
Alternative to Gamunex 10% in Ukraine
Online doctors for Gamunex 10%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Gamunex 10% – subject to medical assessment and local rules.














