
Icatibant Zentiva

Ask a doctor about a prescription for Icatibant Zentiva

How to use Icatibant Zentiva
Leaflet accompanying the packaging: information for the user
Icatibant Zentiva 30 mg solution for injection in a pre-filled syringe
Icatibant
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for one person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Icatibant Zentiva and what is it used for
- 2. Important information before using Icatibant Zentiva
- 3. How to use Icatibant Zentiva
- 4. Possible side effects
- 5. How to store Icatibant Zentiva
- 6. Contents of the packaging and other information
1. What is Icatibant Zentiva and what is it used for
Icatibant Zentiva contains the active substance icatibant.
This medicine is used to treat symptoms of hereditary angioedema (HAE) in adult patients and adolescents and children aged 2 years and above.
In HAE, there is an increase in the blood level of a substance called bradykinin, which leads to symptoms such as swelling, pain, nausea, and diarrhea.
Icatibant Zentiva blocks the activity of bradykinin, thereby interrupting the development of HAE attack symptoms.
2. Important information before using Icatibant Zentiva
When not to use Icatibant Zentiva
- if the patient is allergic to icatibant or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Icatibant Zentiva, the patient should discuss it with their doctor:
- if the patient has angina pectoris (reduced blood flow to the heart muscle);
- if the patient has recently had a stroke.
Side effects related to the use of Icatibant Zentiva are similar to the symptoms of the disease.
If the patient notices worsening of attack symptoms after receiving the medicine, they should immediately inform their doctor.
In addition:
- Before self-administering the medicine or before administering the medicine by a caregiver, the patient or caregiver should be trained in performing subcutaneous injections.
- A patient with a laryngeal attack (upper airway obstruction) who self-administers Icatibant Zentiva or has it administered by a caregiver should seek medical help immediately in a healthcare facility.
- If, after a single self-administration of the medicine or a single administration by a caregiver, the symptoms do not subside, the patient should consult a doctor for further injection of the medicine. Adult patients should not receive more than two additional injections within 24 hours.
Children and adolescents
The use of this medicine is not recommended in children under 2 years of age or weighing less than 12 kg, as it has not been studied in this age group.
Icatibant Zentiva and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
No interactions between Icatibant Zentiva and other medicines are known. If the patient is taking a medicine known as an angiotensin-converting enzyme (ACE) inhibitor (e.g., captopril, enalapril, ramipril, quinapril, lisinopril), used to lower blood pressure or for any other reason, they should inform their doctor before using this medicine.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before starting treatment with this medicine.
The patient should not breastfeed for 12 hours after receiving this medicine.
Driving and using machines
The patient should not drive or use machines if they experience fatigue or dizziness due to an HAE attack or after using Icatibant Zentiva.
Icatibant Zentiva contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means it is considered "sodium-free".
3. How to use Icatibant Zentiva
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor.
If the patient is receiving Icatibant Zentiva for the first time, the first dose of the medicine is always administered by a doctor or nurse. The doctor will tell the patient when they can safely go home.
After discussing with the doctor or nurse and being trained in performing subcutaneous injections, the patient may self-administer Icatibant Zentiva or have it administered by a caregiver if they experience an HAE attack.
It is essential to administer Icatibant Zentiva subcutaneously as soon as possible after noticing an HAE attack. The treating doctor will teach the patient and their caregiver how to safely administer the medicine according to the instructions provided in the "Patient Leaflet".
When and how often to use Icatibant Zentiva
The doctor will determine the exact dose of the medicine and tell the patient how often to use it.
Adults
- The recommended dose of Icatibant Zentiva is one injection (3 mL, 30 mg), administered subcutaneously immediately after noticing an HAE attack (e.g., severe skin swelling, especially on the face and neck, or abdominal pain).
- If symptoms do not subside after 6 hours, the patient should consult their doctor about administering another dose of Icatibant Zentiva. Adult patients should not receive more than two injections within 24 hours.
- Do not take more than 3 injections within 24 hours, and if the patient requires more than 8 injections per month, they should consult their doctor.
Children and adolescents aged 2 to 17 years
- The recommended dose of Icatibant Zentiva is one injection of 1 mL to a maximum of 3 mL, depending on body weight, administered subcutaneously immediately after noticing symptoms of an HAE attack (e.g., severe skin swelling, especially on the face and neck, or abdominal pain).
- For more information on dosing, see "Detailed injection instructions".
- If the patient is unsure about the volume of the solution to be administered, they should consult their doctor, pharmacist, or nurse.
- If symptoms worsen or do not subside, the patient should seek medical help immediately.
How to administer Icatibant Zentiva
Icatibant Zentiva is intended for subcutaneous administration. Each syringe should be used only once.
The medicine is administered using a short needle into the fatty tissue under the skin of the abdomen.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
Detailed injection instructions
- Self-administration (adults)
- Administration by a caregiver or healthcare professional to adults, adolescents, and children over 2 years of age (with a body weight of 12 kg or more).
The instructions include the following main steps:
- 1) General information 2a) Preparing the pre-filled syringe for children and adolescents (2-17 years) with a body weight of 65 kg or less 2b) Preparing the pre-filled syringe and needle for injection (all patients)
- 3) Preparing the injection site
- 4) Injecting the solution
- 5) Disposing of injection equipment
Detailed injection instructions
- 1) General information
- Before starting, clean the surface to be used.
- Wash hands with water and soap.
- Open the packaging by tearing off the protective layer.
- Remove the pre-filled syringe from the packaging.
- Remove the cap from the tip of the pre-filled syringe by twisting it.
- After removing the cap, put the pre-filled syringe aside.
2a) Preparing the pre-filled syringe for children and adolescents (aged 2 to 17 years) with a body weight of 65 kg or less:
Important information for healthcare professionals and caregivers:
If the dose is less than 30 mg (3 mL), to obtain the correct dose from the pre-filled syringe, the following will be needed:
a)
a pre-filled syringe with Icatibant Zentiva (containing icatibant solution)
b)
an adapter
c)
a 3 mL syringe with graduations
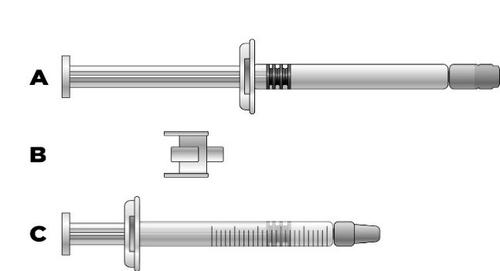
The required dose volume in milliliters should be drawn into an empty 3 mL syringe with graduations (see the table below).
Table 1: Administration scheme for children and adolescents
Patients with a body weight over 65 kgshould receive the entire contents of the pre-filled syringe (3 mL).
| Body weight | Volume of solution |
| 12 kg to 25 kg | 1.0 mL |
| 26 kg to 40 kg | 1.5 mL |
| 41 kg to 50 kg | 2.0 mL |
| 51 kg to 65 kg | 2.5 mL |

If the patient is unsure about the volume of the solution to be administered, they should consult their doctor, pharmacist, or nurse.
- 1) Remove the protective sleeve from both sides of the adapter.

Avoid touching the tip and nozzle of the adapter and syringe to prevent contamination.
- 2) Screw the adapter onto the pre-filled syringe.
- 3) Attach the syringe with graduations to the other end of the adapter, ensuring both ends are securely connected.

- 1) To draw the dose of icatibant solution, press the plunger of the pre-filled syringe (on the left in the illustration below).
- 2) If the icatibant solution does not start flowing into the syringe with graduations, gently pull the syringe plunger until the solution starts flowing (see illustration below).
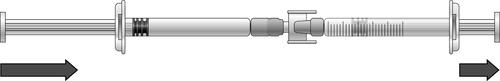
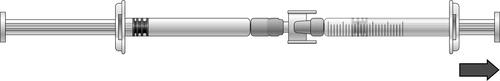
- 3) Continue pressing the plunger of the pre-filled syringe until the required volume of solution (dose) has flowed into the syringe with graduations. Dosing information can be found in Table 1.
If there is air in the syringe with graduations, the following steps should be taken:
- Turn the connected syringes so that the pre-filled syringe is on top.
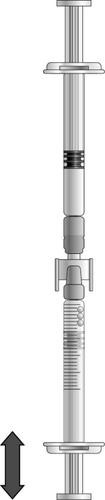
- Press the plunger of the syringe with graduations so that the air returns to the pre-filled syringe (this step may need to be repeated several times).
- Draw the required volume of icatibant solution.
- 4) Disconnect the pre-filled syringe with the adapter from the syringe with graduations.
- 5) Place the pre-filled syringe with the adapter in a special container for sharp objects, intended for disposing of waste that can cause puncture wounds.
2b) Preparing the pre-filled syringe and needle for injection:
All patients (adults, children, and adolescents)
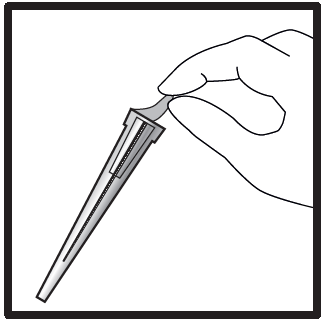
- Remove the needle shield from the packaging.
- Remove the protective layer from the needle shield (the needle should still be in the shield).
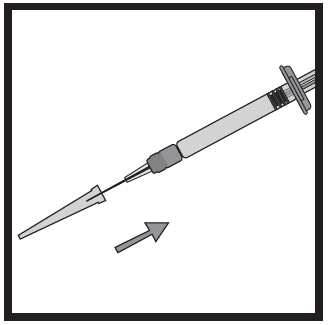
- Firmly hold the pre-filled syringe. Carefully attach the needle to the pre-filled syringe containing the clear solution.
- Screw the pre-filled syringe onto the needle still secured in the shield.
- Remove the needle from the shield by pulling on the pre-filled syringe. Do not pull on the plunger of the pre-filled syringe.
- The pre-filled syringe is now ready for injection .
- 3) Preparing the injection site
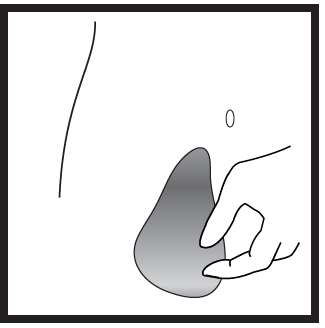
- Choose the injection site. The injection site should be a skin fold on the abdomen, about 5-10 cm below the navel, on either side. This area of skin should be at least 5 cm away from any scars. Do not choose an area of skin with bruising, swelling, or pain for injection.
- Clean the injection site by wiping it with a swab soaked in alcohol and let it dry
- 4) Injecting the solution
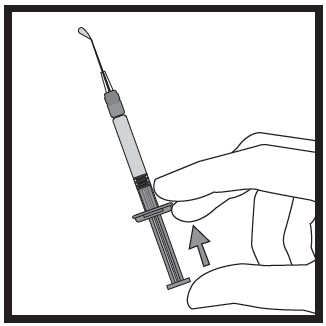
- Hold the pre-filled syringe with one hand, between two fingers, with the thumb on the end of the plunger.
- Make sure there are no air bubbles in the pre-filled syringe by pressing the plunger until the first drop appears at the tip of the needle.
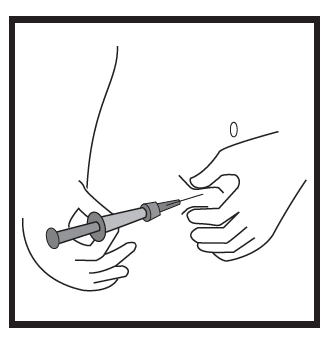
- Hold the pre-filled syringe at an angle of 45-90 degrees to the skin, with the needle pointing towards the skin.
- Holding the pre-filled syringe with one hand, use the other hand to gently grasp the skin fold at the previously cleaned site between the thumb and fingers.
- Hold the skin fold, bring the pre-filled syringe close to the skin, and quickly insert the needle into the skin fold.
- Slowly press the plunger of the pre-filled syringe, keeping the hand still, until the entire fluid has been injected into the skin and the pre-filled syringe is completely empty.
- Press the plunger slowly, over about 30 seconds.
- Release the skin fold and gently pull out the needle.
- 5) Disposing of injection equipment
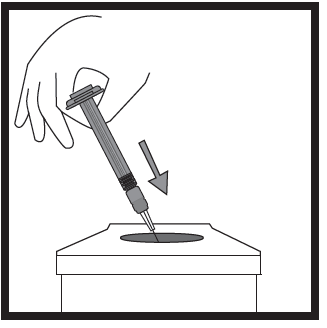
- Place the pre-filled syringe, needle, and needle shield in a container for sharp objects, intended for disposing of waste that can cause puncture wounds.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Almost all patients receiving Icatibant Zentiva experience a reaction at the injection site (irritation, swelling, pain, itching, redness of the skin, and a burning sensation). These reactions are usually mild and resolve without the need for additional treatment.
The patient should immediately inform their doctor if they notice that the attack symptoms worsen after receiving this medicine.
Very common(may occur in more than 1 in 10 patients):
Additional reactions at the injection site (feeling of pressure, bruising, impaired sensation and/or numbness, itchy rash raised above the surrounding skin, and a feeling of warmth).
Common(may occur in up to 1 in 10 patients):
Nausea
Headache
Dizziness
Fever
Itching
Rash
Redness of the skin
Abnormal liver test results
Frequency not known(frequency cannot be estimated from the available data):
Hives
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49-21-301, fax: +48 22 49-21-309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of this medicine.
5. How to store Icatibant Zentiva
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label after: EXP. The expiry date refers to the last day of the month stated.
Do not store above 25°C. Do not freeze.
Do not use this medicine if the syringe or needle packaging is damaged or if there are any visible signs of deterioration, such as if the solution is cloudy, particles are present, or its color has changed.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer use. This will help protect the environment.
6. Contents of the packaging and other information
What Icatibant Zentiva contains
- The active substance is icatibant. Each pre-filled syringe contains 30 milligrams of icatibant (as icatibant acetate). Each milliliter of solution contains 10 mg of icatibant.
- The other ingredients are: sodium chloride, glacial acetic acid, sodium hydroxide (for pH adjustment), and water for injections (see section 2).
What Icatibant Zentiva looks like and contents of the pack
Icatibant Zentiva is a clear, colorless solution for injection in a glass pre-filled syringe with a capacity of 3 mL.
A subcutaneous needle is included with the packaging.
Icatibant Zentiva is available in a single packaging containing one pre-filled syringe with one needle or in a multipack containing three pre-filled syringes with three needles, all in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Zentiva k.s
U kabelovny 130
Dolní Měcholupy
102 37 Prague 10
Czech Republic
Manufacturer/Importer
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park
PLA 3000, Paola, Malta
Eurofins PROXY Laboratories B.V.
Archimedesweg 25
2333 Leiden
Netherlands
To obtain more detailed information about the medicine, the patient should contact the representative of the marketing authorization holder in Poland:
Zentiva Polska Sp. z o.o.
ul. Bonifraterska 17
00-203 Warsaw
tel.: +48 22 375 92 00
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterEurofins Proxy Laboratories BV Pharmadox Healthcare Ltd.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Icatibant ZentivaDosage form: Solution, 30 mgActive substance: icatibantManufacturer: Fresenius Kabi Austria GmbHPrescription requiredDosage form: Solution, 30 mgActive substance: icatibantManufacturer: Universal Farma, S.L.Prescription requiredDosage form: Solution, 30 mgActive substance: icatibantManufacturer: Universal Farma, S.L.Prescription required
Alternatives to Icatibant Zentiva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Icatibant Zentiva in Spain
Alternative to Icatibant Zentiva in Ukraine
Online doctors for Icatibant Zentiva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Icatibant Zentiva – subject to medical assessment and local rules.














