
Icatibant Fresenius

Ask a doctor about a prescription for Icatibant Fresenius

How to use Icatibant Fresenius
Package Leaflet: Information for the User
Icatibant Fresenius, 30 mg, Solution for Injection in a Pre-filled Syringe
Icatibant
Read the Package Leaflet Carefully Before Using the Medicinal Product
- Keep this package leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Icatibant Fresenius and What is it Used For
- 2. Important Information Before Using Icatibant Fresenius
- 3. How to Use Icatibant Fresenius
- 4. Possible Side Effects
- 5. How to Store Icatibant Fresenius
- 6. Contents of the Package and Other Information
1. What is Icatibant Fresenius and What is it Used For
Icatibant Fresenius contains the active substance icatibant.
Icatibant Fresenius is used to treat acute attacks of hereditary angioedema (HAE) in adults and adolescents and children aged 2 years and above.
In HAE, the level of a substance called bradykinin increases in the blood, leading to symptoms such as swelling, pain, nausea, and diarrhea.
Icatibant Fresenius blocks the action of bradykinin, thereby stopping the progression of HAE attack symptoms.
2. Important Information Before Using Icatibant Fresenius
When Not to Use Icatibant Fresenius:
- if you are allergic to icatibant or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
Before starting treatment with Icatibant Fresenius, discuss with your doctor:
- if you have coronary artery disease (reduced blood flow to the heart muscle);
- if you have recently had a stroke.
Some of the side effects of Icatibant Fresenius are similar to the symptoms of the disease.
If you notice an increase in symptoms after receiving Icatibant Fresenius, inform your doctor immediately.
Additionally:
- Before self-administering Icatibant Fresenius or before a caregiver administers Icatibant Fresenius, the patient or caregiver should be trained in performing subcutaneous injections.
- A patient with a laryngeal attack (upper airway obstruction) who self-administers Icatibant Fresenius or has Icatibant Fresenius administered by a caregiver should seek medical attention immediately at a medical facility.
- If symptoms do not resolve after a single self-administered dose of Icatibant Fresenius or a single dose administered by a caregiver, the patient should consult a doctor for a subsequent injection of Icatibant Fresenius. Adult patients should not receive more than two additional injections within 24 hours.
Children and Adolescents
Icatibant Fresenius should not be used in children below 2 years of age or weighing less than 12 kg, as it has not been studied in this age group.
Icatibant Fresenius and Other Medicines
Tell your doctor about all medicines you are taking, have recently taken, or might take.
No interactions of Icatibant Fresenius with other medicines are known. If you are taking an ACE inhibitor (e.g., captopril, enalapril, ramipril, quinapril, lisinopril) for high blood pressure or any other reason, inform your doctor before using Icatibant Fresenius.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
You should not breastfeed for 12 hours after using Icatibant Fresenius.
Driving and Using Machines
Do not drive or use machines if you feel tired or dizzy due to an HAE attack or after using Icatibant Fresenius.
Icatibant Fresenius Contains Sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means it is essentially 'sodium-free'.
3. How to Use Icatibant Fresenius
Use this medicine exactly as your doctor has told you.
If you are using Icatibant Fresenius for the first time, the first dose should always be given by a doctor or nurse. The doctor will tell you when you can safely return home. After discussion with your doctor or nurse and training in performing subcutaneous injections, you may self-administer Icatibant Fresenius or a caregiver may administer Icatibant Fresenius to you if you have an HAE attack.
It is essential to inject Icatibant Fresenius as soon as possible after noticing an HAE attack. Your treating doctor will teach you and your caregiver how to safely inject Icatibant Fresenius according to the instructions in the package leaflet.
When and How Often to Use Icatibant Fresenius
Your doctor will decide the exact dose of Icatibant Fresenius and tell you how often to use it.
Adults
- The recommended dose of Icatibant Fresenius is one injection (3 ml, 30 mg), given subcutaneously as soon as possible after noticing an HAE attack (e.g., severe skin swelling, especially of the face and neck, severe abdominal pain).
- If symptoms do not resolve after 6 hours, consult your doctor for a subsequent injection of Icatibant Fresenius. Adult patients should not receive more than two additional injections within 24 hours.
- Do not take more than 3 injections in 24 hours. If you need more than 8 injections in a month, consult your doctor.
Children and Adolescents Aged 2 to 17 Years
- The recommended dose of Icatibant Fresenius is one injection of 1 ml to a maximum of 3 ml, depending on body weight, given subcutaneously as soon as possible after noticing symptoms of an HAE attack (e.g., severe skin swelling, especially of the face and neck, severe abdominal pain).
- For more information on dosing, see "Detailed Injection Instructions".
- If you are unsure about the dose to administer, consult your doctor, pharmacist, or nurse.
- If symptoms worsen or do not resolve, seek medical attention immediately.
How to Administer Icatibant Fresenius
Icatibant Fresenius is for subcutaneous use. Each pre-filled syringe is for single use only.
Icatibant Fresenius is injected into the fatty tissue under the skin of the abdomen using a short needle.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
The Following Detailed Instructions Apply To:
- Self-administration (adults);
- Administration by a caregiver or medical professional to adults, adolescents, and children over 2 years of age (with a body weight of 12 kg or more).
The instructions include the following main steps:
- 1) General Information 2a) Preparing the Pre-filled Syringe for Children and Adolescents (Aged 2 to 17 Years) with a Body Weight of 65 kg or Less 2b) Preparing the Pre-filled Syringe and Needle for Injection (All Patients)
- 3) Preparing the Injection Site
- 4) Injecting the Solution
- 5) Disposing of the Injection Equipment
Detailed Injection Instructions
- 1) General Information
- Before starting, clean the surface you are using.
- Wash your hands with water and soap.
- Open the packaging by tearing off the protective strip.
- Remove the pre-filled syringe from the packaging.
- Remove the cap from the end of the pre-filled syringe.
- After removing the cap, put the pre-filled syringe down.
2a) Preparing the Pre-filled Syringe for Children and Adolescents (Aged 2 to 17 Years) with a Body Weight of 65 kg or Less
Important Information for Healthcare Professionals and Caregivers:
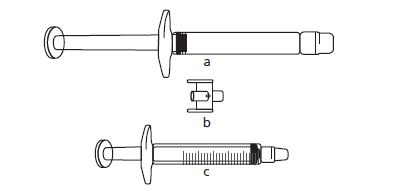
If the dose is less than 30 mg (3 ml), to obtain the correct dose from the pre-filled syringe, you will need (see below):
a) the Icatibant Fresenius pre-filled syringe (containing the icatibant solution),
b) an adapter (connector),
c) a 3 ml syringe with graduations.
Draw the required volume of solution in milliliters into the empty 3 ml syringe with graduations (see the table below).
Table 1. Administration Schedule for Children and Adolescents
Body Weight Volume of Solution
12 kg to 25 kg
1.0 ml
26 kg to 40 kg
1.5 ml
41 kg to 50 kg
2.0 ml
51 kg to 65 kg
2.5 ml
Patients with a body weight above 65 kgshould receive the entire contents of the pre-filled syringe (3 ml).
If you are unsure about the volume of solution to draw up, consult your doctor, pharmacist, or nurse.
- 1) Remove the caps from both ends of the adapter.

Avoid touching the end and tip of the adapter and syringe to prevent contamination.
- 2) Screw the adapter onto the pre-filled syringe.

- 3) Attach the syringe with graduations to the other end of the adapter, ensuring both ends are securely connected.
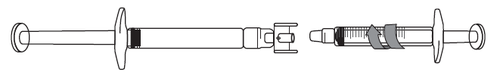
Withdrawing the Icatibant Solution into the Syringe:
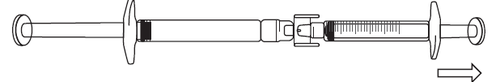
- 1) To start withdrawing the solution, press the plunger of the pre-filled syringe (on the left in the illustration below).
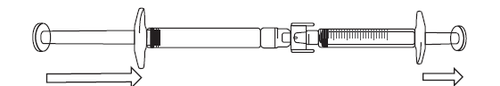
- 2) If the solution does not start flowing into the syringe with graduations, gently pull the plunger of the syringe until the solution starts to flow in (see the illustration below).
- 3) Continue pressing the plunger of the pre-filled syringe until the required volume of solution (dose) has been transferred to the syringe with graduations. The dosing information can be found in Table 1.
If there is air in the syringe, do the following:
- Turn the connected syringes so that the pre-filled syringe is on top (see the illustration).
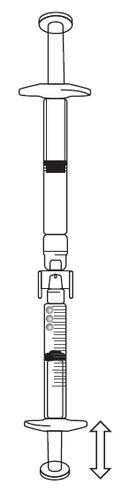
- Press the plunger of the syringe with graduations to return the air to the pre-filled syringe (this may need to be repeated several times).
- Withdraw the required volume of solution.
- 4) Disconnect the pre-filled syringe with the adapter from the syringe with graduations.
- 5) Place the pre-filled syringe with the adapter in a sharps container.
2b) Preparing the Pre-filled Syringe and Needle for Injection
All Patients (Adults, Adolescents, and Children)
- Remove the needle from its packaging.
- Remove the protective cap from the needle (the needle should still be in its protective sleeve).
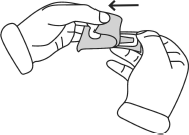
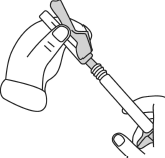
- Firmly hold the syringe. Carefully attach the needle to the syringe containing the colorless solution, twisting it firmly in a clockwise direction.
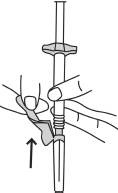
- Remove the needle guard in the direction of the syringe, away from the needle.
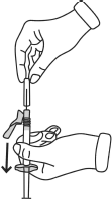
- Remove the needle from its cap by pulling on the syringe body. Do not pull on the plunger.
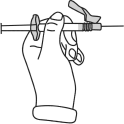
- The syringe is now ready for injection.
- 3) Preparing the Injection Site
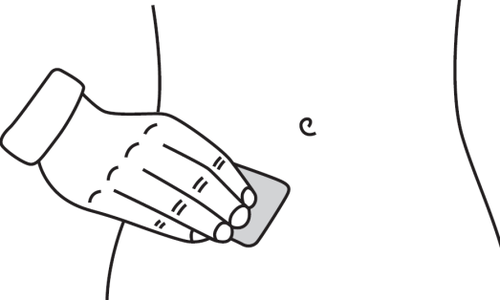
- Choose an injection site. The injection site should be a skin fold on the abdomen, about 5-10 cm below the navel, on either side. This area of skin should be at least 5 cm away from any scars. Do not choose an area of skin with bruising, swelling, or pain for injection.
- Clean the injection site by wiping it with a gauze pad moistened with alcohol and let it dry.
- 4) Injecting the Solution
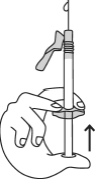
- Hold the syringe with one hand, between two fingers, with your thumb on the end of the plunger.
- Make sure there are no air bubbles in the syringe by pressing the plunger until the first drop appears at the tip of the needle.
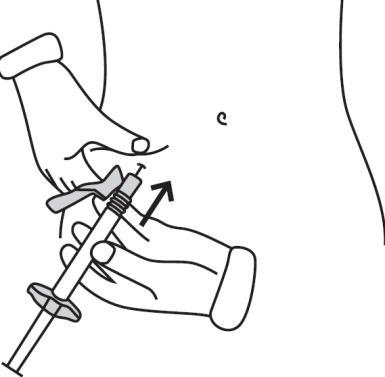
- Hold the syringe at an angle of 45-90 degrees to the skin, with the needle pointing towards the skin.
- Holding the syringe with one hand, use the other hand to gently grasp the skin fold between your thumb and fingers at the previously cleaned site.
- Hold the skin fold, bring the syringe close to the skin, and quickly insert the needle into the skin fold.
- Slowly press the plunger of the syringe, keeping your hand still, until the entire liquid has been injected into the skin and the syringe is empty.
- Press the plunger slowly, over about 30 seconds.
- Release the skin fold and gently withdraw the needle.
- 5) Disposing of the Injection Equipment
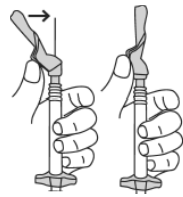
- Slide the needle guard forward over the needle until you hear and feel it click.
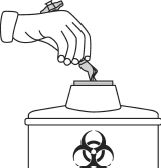
- Place the syringe, needle, and needle cap in a sharps container, intended for disposing of sharp objects that can cause injury.
4. Possible Side Effects
Like all medicines, Icatibant Fresenius can cause side effects, although not everybody gets them.
Almost all patients treated with Icatibant Fresenius experience an injection site reaction (irritation, swelling, pain, itching, redness of the skin, and a burning sensation). These reactions are usually mild and resolve without the need for additional treatment.
Very Common (May Affect More Than 1 in 10 People):
Additional injection site reactions (feeling of pressure, bruising, impaired sensation, and/or numbness, raised, itchy rash on the skin, and a feeling of warmth).
Common (May Affect Up to 1 in 10 People):
- nausea;
- headache;
- dizziness;
- fever;
- itching;
- rash;
- redness of the skin;
- abnormal liver function test results.
Frequency Not Known (Cannot be Estimated from the Available Data):
- hives.
Inform your doctor immediately if you notice an increase in symptoms or worsening of the disease after using Icatibant Fresenius.
If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist.
Reporting Side Effects
If you experience any side effects, including those not listed in this package leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to Store Icatibant Fresenius
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and pre-filled syringe after EXP. The expiry date refers to the last day of that month.
Do not store above 30°C. Do not freeze.
Use immediately after opening and use only undamaged packaging.
For single use only.
Do not use this medicine if the pre-filled syringe or needle packaging is damaged or if there are any visible signs of deterioration, such as the solution being cloudy, containing particles, or having changed color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the Package and Other Information
What Icatibant Fresenius Contains
- The active substance is icatibant. Each pre-filled syringe contains 30 mg of icatibant (as icatibant acetate). Each milliliter of solution contains 10 mg of icatibant.
- The other ingredients are sodium chloride, acetic acid, sodium hydroxide (for pH adjustment), and water for injections.
What Icatibant Fresenius Looks Like and Contents of the Package
Icatibant Fresenius is a clear, colorless solution for injection in a pre-filled syringe (made of type I glass) with a capacity of 3 ml, with a plunger stopper made of bromobutyl rubber coated with a fluoropolymer, in a cardboard box.
A subcutaneous needle with a safety device (25G; 16 mm) is included with the packaging.
Icatibant Fresenius is available in a package containing one pre-filled syringe with one needle with a safety device or three pre-filled syringes with three needles with safety devices.
Not all pack sizes may be marketed.
Marketing Authorization Holder
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
Manufacturer
Fresenius Kabi Austria GmbH
Hafnerstraße 36
A-8055 Graz
Austria
For more detailed information, please contact the marketing authorization holder:
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
tel.: +48 22 345 67 89
This Medicinal Product is Authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) Under the Following Names:
| Member State | Medicinal Product Name |
| Austria | Icatibant Fresenius 30 mg Injektionslösung in einer Fertigspritze |
| Belgium | Icatibant Fresenius 30 mg oplossing voor injectie in een voorgevulde spuit, solution injectable en seringue pré-remplie, Injektionslösung in einer Fertigspritze |
| Croatia | Ikatibant Fresenius 30 mg otopina za injekciju u napunjenoj štrcaljki |
| Czech Republic | Icatibant Fresenius |
| Denmark | Icatibant Fresenius |
| Estonia | Icatibant Fresenius |
| Finland | Icatibant Fresenius 30 mg injektioneste, liuos, esitäytetty ruisku |
| France | ICATIBANT FRESENIUS 30 mg, solution injectable en seringue préremplie |
| Germany | Icatibant Fresenius 30 mg Injektionslösung in einer Fertigspritze |
| Hungary | Icatibant Fresenius 30 mg oldatos injekció előretöltött fecskendőben |
| Ireland | Icatibant 30 mg solution for injection in pre-filled syringe |
| Italy | Icatibant Fresenius |
| Latvia | Icatibant Fresenius 30 mg šķīdums injekcijām pilnšļircē |
| Lithuania | Icatibant Fresenius 30 mg injekcinis tirpalas užpildytame švirkšte |
| Netherlands | Icatibant Fresenius 30 mg, oplossing voor injectie in een voorgevulde spuit |
| Norway | Icatibant Fresenius |
| Poland | Icatibant Fresenius |
| Portugal | Icatibant Fresenius |
| Slovakia | Icatibant Fresenius 30 mg |
| Spain | Icatibanto Fresenius 30 mg solución inyectable en jeringa precargada EFG |
| Sweden | Icatibant Fresenius 30 mg injektionsvätska, lösning i förfylld spruta |
| United Kingdom (Northern Ireland) | Icatibant 30 mg solution for injection in pre-filled syringe |
Date of Last Revision of the Package Leaflet:15.04.2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFresenius Kabi Austria GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Icatibant FreseniusDosage form: Solution, 30 mgActive substance: icatibantManufacturer: Universal Farma, S.L.Prescription requiredDosage form: Solution, 30 mgActive substance: icatibantManufacturer: Universal Farma, S.L.Prescription requiredDosage form: Solution, 30 mgActive substance: icatibantManufacturer: Eurofins Proxy Laboratories BV Pharmadox Healthcare Ltd.Prescription required
Alternatives to Icatibant Fresenius in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Icatibant Fresenius in Spain
Alternative to Icatibant Fresenius in Ukraine
Online doctors for Icatibant Fresenius
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Icatibant Fresenius – subject to medical assessment and local rules.














