
Hipnomidate

Ask a doctor about a prescription for Hipnomidate

How to use Hipnomidate
Leaflet accompanying the packaging: information for the user
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Hypnomidate, 2 mg/ml, solution for injection
Etomidate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Hypnomidate and what is it used for
- 2. Important information before using Hypnomidate
- 3. How to use Hypnomidate
- 4. Possible side effects
- 5. How to store Hypnomidate
- 6. Contents of the packaging and other information
1. What is Hypnomidate and what is it used for
The active substance of Hypnomidate is etomidate. Hypnomidate is indicated for the induction of general anesthesia, as well as for supplementary anesthesia in local anesthesia. Hypnomidate is indicated for short diagnostic procedures or procedures performed on an outpatient basis, where rapid recovery of the patient is necessary. Hypnomidate is particularly indicated in cardio surgery and in patients with heart disease.
2. Important information before using Hypnomidate
When not to use Hypnomidate
If the patient is allergic to the active substance or any of the other ingredients of this medicine (listed in section 6). An allergic reaction can be recognized as: rash, itching, swelling of the face and lips, shortness of breath. All such reactions should be immediately reported to the doctor.
Warnings and precautions
The medicine should be used with caution in patients:
- with adrenal insufficiency, such as in patients with sepsis;
- with liver cirrhosis;
- who have received neuroleptic drugs, opioids, or sedatives (see below - Hypnomidate and other medicines);
- of advanced age.
Single inducing doses of etomidate may lead to transient adrenal insufficiency and decreased cortisol levels in the serum. Etomidate should be used with caution in critically ill patients, including those with sepsis, as it has been associated with an increased risk of death in some studies. Hypnomidate must be administered only by doctors trained in the administration of endotracheal intubation. Resuscitation equipment must be available in case of respiratory depression and apnea. The induction of general anesthesia with Hypnomidate may be accompanied by a slight, transient decrease in blood pressure. In weakened patients, the doctor will take appropriate measures. The use of Hypnomidate may be associated with the occurrence of spontaneous muscle movements, especially if no premedication (administration of a sedative before administration of the anesthetic) has been used. Hypnomidate does not have analgesic properties, and therefore, an appropriate analgesic should be administered during the surgical procedure - 1-2 minutes before the administration of Hypnomidate.
Hypnomidate and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as medicines that are available without a prescription. The effect of Hypnomidate may be increased by:
- neuroleptic drugs - used to treat mental illnesses,
- opioids - painkillers, e.g., morphine, fentanyl,
- sedatives,
- alcohol. Hypnomidate may enhance the effect of:
- drugs that lower blood pressure.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, or thinks she may be pregnant or plans to have a child, she should consult a doctor before using this medicine. The doctor will then decide whether to use Hypnomidate. During treatment and for 24 hours after using Hypnomidate, breastfeeding should not be performed. Reproductive studies in animals have not shown that Hypnomidate, when used in recommended doses, affects fertility.
Driving and using machines
Hypnomidate has a significant impact on the ability to drive vehicles and operate machines. It is not recommended to operate potentially hazardous machines and drive vehicles for the first 24 hours after administration. The time to return to normal fitness may vary depending on the duration of the procedure, the total dose administered, and other medications used. Therefore, the decision to allow the patient to drive vehicles and operate machines must be made by the anesthesiologist.
Hypnomidate contains propylene glycol
The medicine contains 3.626 g of propylene glycol in each ampoule. Before administering the medicine to a child under 5 years of age, you should consult a doctor or pharmacist, especially if the child is taking other medicines containing propylene glycol or alcohol. Pregnant or breastfeeding women should not take this medicine without a doctor's recommendation. The doctor may decide to perform additional tests on such patients. Patients with liver or kidney function disorders should not take this medicine without a doctor's recommendation. The doctor may decide to perform additional tests on such patients.
3. How to use Hypnomidate
Hypnomidate is intended for administration only by medical personnel. In case of doubts, you should consult a doctor.
How to administer Hypnomidate
- only intravenously - in slow injection or infusion;
- it can be diluted with physiological sodium chloride solution or glucose solution.
How much Hypnomidate to administer
The amount of medicine used is decided by the doctor, depending on:
- the type of surgical procedure,
- body weight,
- age,
- general health. The effective dose inducing sleep is 0.3 mg/kg body weight. Therefore, in adult patients, one ampoule usually suffices to induce sleep lasting 4-5 minutes. Sleep can be prolonged by administering additional doses of Hypnomidate, not more than 3 ampoules (30 ml).
Elderly patients
Elderly patients are given a single dose of 0.15-0.2 mg/kg body weight, which is then individually adjusted depending on the patient's reaction (see section 2. Important information before using Hypnomidate).
Children
In children under 15 years of age, it may be necessary to increase the dose in order to achieve the same depth of sleep and sleep time as in adults. Sometimes it is necessary to increase the dose by about 30% of the usual dose for adults.
Using a higher dose of Hypnomidate than recommended
In the event of an overdose of etomidate administered as a bolus, sleep becomes deeper and may be accompanied by hypotension, inhibition of adrenal cortical function, respiratory depression, or even respiratory arrest. In case of respiratory arrest, appropriate supportive measures should be taken. Disorientation and prolonged recovery may also occur.
4. Possible side effects
Like all medicines, Hypnomidate can cause side effects, although not everybody gets them. In clinical trials, the most commonly reported side effects were dyskinesia and vein pain. The following side effects (including those listed above) were observed during clinical trials or after the marketing of Hypnomidate.
- Very common side effects (may occur in at least 1 in 10 patients):
- dyskinesia (movement disorder);
- decreased cortisol levels.
Common side effects (may occur less often than 1 in 10 patients):
- myoclonic seizures;
- vein pain, decreased blood pressure;
- apnea, hyperventilation (acceleration or deepening of breathing), stridor;
- nausea, vomiting;
- rash.
Uncommon side effects (may occur less often than 1 in 100 patients):
- hypertonia (increased muscle tension), involuntary muscle contractions, oculogyric crisis;
- bradycardia (slow heart rate), extra heartbeats, ventricular extra beats;
- phlebitis, hypertension (high blood pressure);
- hypoventilation (slowing and weakening of breathing), hiccups, cough;
- excessive salivation;
- flushing;
- muscle stiffness;
- pain at the injection site;
- anesthesia complications, delayed recovery of consciousness after anesthesia, insufficient analgesic effect, nausea during procedures.
Side effects with unknown frequency (frequency cannot be estimated from available data):
- hypersensitivity reactions (including anaphylactic shock), anaphylactic reactions (severe allergic reactions causing breathing difficulties or dizziness) and pseudo-anaphylactic reactions;
- adrenal insufficiency;
- seizures (including grand mal seizures);
- cardiac arrest, complete atrioventricular block;
- shock, thrombophlebitis (deep and superficial);
- respiratory depression, bronchospasm (including death);
- Stevens-Johnson syndrome (characterized by non-permanent blisters on mucous membranes, mainly in the mouth and genital organs, which burst to form painful ulcers that make eating difficult). It may also be accompanied by fever, joint pain, urticaria;
- trismus.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Hypnomidate
Do not store above 25°C. The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated. Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Hypnomidate contains
- The active substance of Hypnomidate is etomidate. Hypnomidate ampoules contain 10 ml (20 mg etomidate) of ready-to-use solution (2 mg etomidate per 1 ml of solution).
- The other excipients are propylene glycol and water for injections.
What Hypnomidate looks like and what the packaging contains
A 10 ml ampoule containing a sterile, clear, colorless aqueous solution for intravenous injection. 1 package contains 5 ampoules of 10 ml each. For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Germany, the country of export:
Piramal Critical Care B.V., Rouboslaan 32, 2252 TR Voorschoten, Netherlands
Manufacturer:
Piramal Critical Care B.V., Rouboslaan 32, 2252 TR Voorschoten, Netherlands
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź, German authorization number: 319.00.00
Parallel import authorization number: 167/23
Date of leaflet approval: 23.08.2023
[Information about the trademark]
-------------------------------------------------------------------------------------------------------------------------
THE FOLLOWING INFORMATION IS INTENDED EXCLUSIVELY FOR HEALTHCARE PROFESSIONALS
This medicine must be administered only by doctors trained in the administration of endotracheal intubation. When using Hypnomidate, resuscitation equipment should be available in case of respiratory depression and apnea.
1. Preparation of the medicine for use
Before opening the ampoule, protective gloves should be put on.
Ampoules
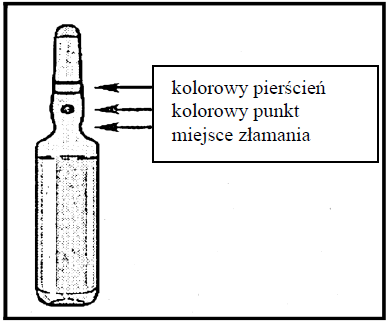
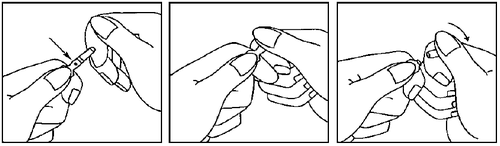
- 1. Hold the ampoule between the thumb and index finger, leaving the free end of the ampoule.
- 2. With the other hand, hold the end of the ampoule, placing the index finger at the end of the ampoule and the thumb on the colored dot, parallel to the colored ring on the end of the ampoule.
- 3. Holding the thumb on this point, break the end of the ampoule energetically, while keeping the rest of the ampoule firmly in the other hand.
In case of accidental exposure of the skin to the product, the area should be washed with water. You should avoid using soap, alcohol, and other cleaning agents that may cause chemical or mechanical damage to the skin.
2. Special warnings and precautions for use
Hypnomidate should be administered only intravenously. The induction of anesthesia with Hypnomidate may be accompanied by a slight, transient decrease in blood pressure, related to the decrease in peripheral vascular resistance. In weakened patients, in whom hypotension may be dangerous, the following measures should be taken:
- 1. The patient to be anesthetized should lie on their back.
- 2. Access to a vein should be obtained to ensure adequate blood volume.
- 3. Hypnomidate should be injected slowly (e.g., 10 ml over 1 minute).
- 4. If possible, the use of other medicines for induction of anesthesia should be avoided.
When using Hypnomidate, resuscitation equipment should be available in case of respiratory depression and apnea. The use of Hypnomidate may be associated with the occurrence of spontaneous muscle movements or muscle groups, especially if no premedication has been used. These movements are attributed to subcortical disinhibition. They can usually be prevented by administering small doses of fentanyl with diazepam intravenously 1-2 minutes before the induction of anesthesia with Hypnomidate. During the administration of Hypnomidate, myoclonic seizures and pain at the injection site have been observed, especially during injection into small veins. This can usually be prevented by administering a small dose of an appropriate opioid, e.g., fentanyl, intravenously 1-2 minutes before the induction of anesthesia. Caution should be exercised when administering Hypnomidate to elderly patients, as it may decrease cardiac minute volume, which has been observed when administering doses higher than recommended. Hypnomidate does not have analgesic properties, and therefore, an appropriate analgesic should be administered during the surgical procedure. Neuroleptic drugs, opioids, sedatives, and alcohol may increase the sedative effect of etomidate. The induction of etomidate may be accompanied by a slight and transient decrease in peripheral resistance, which may enhance the effect of other drugs that lower blood pressure.
Effect of other medicines on etomidate
The concurrent administration of etomidate with alfentanil reduces the terminal half-life of etomidate to 29 minutes. Caution should be exercised when using etomidate and alfentanil concurrently, as the etomidate concentration may fall below the sedative threshold. The total clearance and volume of distribution of etomidate are reduced 2 to 3 times without affecting the half-life when administered with fentanyl intravenously. There may be a need to reduce the dose when etomidate is administered concurrently with fentanyl intravenously.
Effect of etomidate on other medicines
The concurrent administration of etomidate and ketamine does not have a significant effect on the serum concentration or pharmacokinetic parameters of ketamine or its main metabolite, norketamine.
3. Overdose
In the event of an overdose of etomidate administered as a bolus, sleep becomes deeper and may be accompanied by hypotension, inhibition of adrenal cortical function, respiratory depression, or even respiratory arrest. In case of respiratory arrest, appropriate supportive measures should be taken. Disorientation and prolonged recovery may also occur. In addition to supportive measures (e.g., breathing), it may be necessary to administer 50 mg-100 mg of hydrocortisone (and not adrenocorticotropic hormone - ACTH).
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Piramal Critical Care B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HipnomidateDosage form: Emulsion, 2 mg/mlActive substance: etomidateManufacturer: B. Braun Melsungen AGPrescription not requiredDosage form: Solution, 2 mg/mlActive substance: etomidateManufacturer: Piramal Critical Care B.V.Prescription not requiredDosage form: Solution, 2 mg/mlActive substance: etomidatePrescription not required
Alternatives to Hipnomidate in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Hipnomidate in Spain
Online doctors for Hipnomidate
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Hipnomidate – subject to medical assessment and local rules.














