
Fraxiparine

Ask a doctor about a prescription for Fraxiparine

How to use Fraxiparine
1. What is Fraxiparine and what is it used for
Fraxiparine is a medicine that helps prevent the formation of blood clots in blood vessels or treats existing clots. Medicines of this type are called anticoagulants.
The medicine is administered by subcutaneous or intravenous injection.
Indications for use:
- prevention of venous thromboembolic disease in surgery and orthopedic surgery;
- prevention of venous thromboembolic disease in immobilized patients for reasons other than surgery, who have a high risk of thromboembolic complications (e.g. severe chronic obstructive pulmonary disease, heart failure, severe infections, etc.);
- prevention of clotting in the extracorporeal circulation system during hemodialysis;
- treatment of deep vein thrombosis with or without pulmonary embolism;
- treatment of unstable angina and myocardial infarction without Q-wave, in combination with acetylsalicylic acid.
2. Important information before using Fraxiparine
When not to use Fraxiparine
- if the patient is allergic to the active substance nadroparin calcium, heparin or a similar product (such as enoxaparin, bemiparin, dalteparin) or any of the other ingredients of this medicine (listed in section 6);
- if the patient has a history of decreased platelet count (thrombocytopenia) after using Fraxiparine;
- if the patient has bleeding or any disease that reduces blood clotting;
- if the patient has a medical condition associated with an increased risk of bleeding [e.g. active gastric or duodenal ulcer];
- if the patient has recently had a hemorrhagic stroke;
- if the patient has acute infectious endocarditis;
- if the patient has severe kidney dysfunction and is taking anticoagulant medications, e.g. for pulmonary embolism or deep vein thrombosis;
- if the patient has severe kidney dysfunction and is taking medications for heart disease (e.g. unstable angina or myocardial infarction without Q-wave).
Warnings and precautions
During treatment with Fraxiparine, a decrease in platelet count may rarely occur, which can sometimes be severe. During treatment, the doctor will prescribe blood tests to determine if such an effect occurs.
Before using Fraxiparine, the patient should tell their doctor if:
- the patient has conditions that increase the risk of bleeding, in particular, if:
- the patient has gastric or duodenal ulcer disease,
- the patient has coagulation disorders,
- the patient has recently undergone surgery in the brain, spinal cord, or eye,
- the patient has high blood pressure;
- the patient has severe liver dysfunction;
- the patient has kidney dysfunction;
- the patient has eye diseases involving blood vessels (vascular disorders of the retina and choroid);
- the patient is taking other medications that affect blood clotting (see "Fraxiparine and other medications").
In case of bleeding, you should immediately contact your doctor.
In case of skin necrosis, which may be preceded by small subcutaneous hemorrhages or hard or painful redness of the skin, you should immediately stop taking the medicine and contact your doctor.
Using Fraxiparine may cause an increase in potassium levels in the blood. If the patient has diseases in which an increase in potassium levels may occur, such as diabetes, severe kidney disease, previously occurring metabolic acidosis, or if the patient is taking other medications that increase potassium levels in the blood, the doctor may prescribe control blood tests. If the patient is not sure if they are taking such medications, they should ask their doctor.
If the patient is undergoing spinal or epidural anesthesia, or if cerebrospinal fluid is being drawn (lumbar puncture), there is a risk of bleeding into the spinal cord at the injection site, which can lead to serious consequences. During such procedures, the patient's condition will be frequently monitored to determine if any worrying symptoms occur.
If the patient is elderly (over 65 years old), the doctor may prescribe blood tests before starting treatment with Fraxiparine.
The needle shield of the pre-filled syringe may contain latex [see "The packaging of the medicine contains latex (natural rubber)"].
Fraxiparine and other medications
The patient should tell their doctor or pharmacist about all medications they are currently taking or have recently taken, as well as any medications they plan to take.
It is not recommended to use Fraxiparine at the same time as the following medications due to the risk of bleeding. If this cannot be avoided, the doctor will closely monitor the patient's condition. This applies to the following medications:
- acetylsalicylic acid (e.g. aspirin),
- non-steroidal anti-inflammatory drugs (NSAIDs) (medications used to relieve pain, e.g. ibuprofen),
- antiplatelet agents (used to prevent blood clots, e.g. clopidogrel).
Fraxiparine should be used with caution with:
- oral anticoagulants (medications that prevent blood clotting, e.g. warfarin),
- glucocorticosteroids (steroid medications used to treat e.g. asthma),
- dextran (a medication administered intravenously to increase blood volume).
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Fraxiparine may be used during pregnancy only if the doctor considers that the expected benefits of using the medicine outweigh the potential risks.
It is not known whether the ingredients of Fraxiparine pass into breast milk, so breastfeeding should not be done during treatment with Fraxiparine.
There is no data on the effect of nadroparin on fertility.
Driving and using machines
There is no data on the effect of Fraxiparine on the ability to drive and use machines.
The packaging of the medicine may contain latex (natural rubber)
The packaging of the medicine (needle shield) may contain latex. This can cause severe allergic reactions.
3. How to use Fraxiparine
This medicine should always be used as directed by the doctor. If you have any doubts, you should consult a doctor or pharmacist.
Method of administration
Fraxiparine should be administered by subcutaneous or intravenous injection.
Fraxiparine should not be administered by intramuscular injection.
The pre-filled syringe with graduations facilitates the administration of the correct doses, if it is necessary to adjust the dose to the patient's body weight.
In the case of subcutaneous injection, the anterior abdominal wall is usually chosen as the injection site, alternating between the right and left sides. Another injection site may be the thigh.
Detailed instructions for self-administering subcutaneous injections of Fraxiparine can be found in the section "Instructions for self-administering subcutaneous injections of Fraxiparine".
Recommended dosage
The dosage is determined by the doctor, individually for each patient, depending on the indication, clinical condition, and patient's body weight.
Use in children and adolescents
Fraxiparine should not be used in children and adolescents.
Patient over 65 years old (elderly patients)
In these patients, kidney function may be impaired. Therefore, the doctor may prescribe kidney function tests and adjust the dose of Fraxiparine accordingly.
Kidney dysfunction
The doctor will adjust the dose of the medicine according to the severity of the kidney dysfunction.
Fraxiparine is contraindicated in patients with severe kidney dysfunction.
Using a higher dose of Fraxiparine than recommended
In case of using a dose of the medicine higher than recommended, you should immediately contact your doctor. The main symptom of overdose is bleeding.
Missing a dose of Fraxiparine
You should continue using the medicine, without increasing the next dose. You should not shorten the time interval between consecutive doses.
You should not take a double dose to make up for a missed dose.
Stopping treatment with Fraxiparine
Fraxiparine should be used for as long as the doctor recommends. You should not stop using Fraxiparine without consulting your doctor.
If the patient wants to stop using Fraxiparine, they should discuss this with their doctor or pharmacist beforehand.
In case of any further doubts related to the use of this medicine, you should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Fraxiparine can cause side effects, although not everybody gets them.
You should immediately stop taking the medicine and contact your doctor if you experience:
- an allergic reactioncharacterized by: hives (light red, itchy blisters on the skin), angioedema (swelling of the face or lips that can make breathing difficult),
- skin necrosis at the injection site -it is preceded by subcutaneous hemorrhages or hard or painful redness of the skin with or without general symptoms.
Very common side effects(may affect more than 1 in 10 people):
- bleeding of various locations, more frequent in patients with other risk factors,
- small hematomas at the injection site. In some cases, hard lumps may appear, which do not indicate heparin crystallization and should disappear after a few days.
Common side effects(may affect up to 1 in 10 people):
- skin reaction at the injection site,
- increased blood levels of liver enzymes (aminotransferases), usually transient (visible in blood test results).
Rare side effects(may affect up to 1 in 1000 people):
- decreased platelet count (thrombocytopenia),
- increased platelet count (thrombocytosis),
- rash, hives, redness, itching of the skin,
- calcification under the skin at the injection site.
Very rare side effects(may affect up to 1 in 10,000 people):
- allergic reactions, including angioedema and skin reactions,
- pseudoanaphylactic reaction (symptoms are similar to anaphylaxis -allergic
- see at the beginning of this section),
- skin necrosis,
- increased eosinophil count (a type of white blood cell) - transient after treatment (visible in blood test results),
- increased potassium levels in the blood,
- prolonged, painful erection of the penis (priapism) - if this occurs, you should urgently contact your doctor, as the patient may require treatment to avoid serious complications.
Unknown(frequency cannot be determined from available data)
- headache,
- migraine.
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Fraxiparine
The medicine should be stored out of sight and reach of children.
Store at a temperature below 30 ° C.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Medicines and syringes should not be disposed of in the sewage system or household waste containers.
You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Fraxiparine contains
- The active substance of the medicine is: 2,850 AXa units of nadroparin calcium in 0.3 ml of solution for injection, 3,800 AXa units of nadroparin calcium in 0.4 ml of solution for injection, 5,700 AXa units of nadroparin calcium in 0.6 ml of solution for injection, 7,600 AXa units of nadroparin calcium in 0.8 ml of solution for injection, 9,500 AXa units of nadroparin calcium in 1 ml of solution for injection
- Other ingredients are: calcium hydroxide solution or diluted hydrochloric acid to adjust pH, water for injections.
What Fraxiparine looks like and what the packaging contains
Fraxiparine is a solution for injection. The pre-filled syringe contains a clear to slightly opalescent, colorless or slightly yellowish or slightly brownish or slightly dark yellowish solution. The medicine is packaged in pre-filled syringes with a protective needle shield.
The packaging contains 2 or 10 pre-filled syringes with a protective needle shield, in individual blisters, in a cardboard box.
Marketing authorization holder
Viatris Healthcare Sp. z o.o.
ul. Postępu 21B
02-676 Warsaw
tel.: 22 546 64 00
Manufacturer
Aspen Notre Dame de Bondeville
1, rue de l’Abbaye
76960 Notre-Dame de Bondeville
France
or
GlaxoSmithKline Pharmaceuticals S.A.
ul. Grunwaldzka 189
60-322 Poznań
Date of last revision of the leaflet: 02/2024
Information for healthcare professionals
Detailed dosing and administration instructions can be found in section 4.2 of the approved Summary of Product Characteristics.
INSTRUCTIONS FOR SELF-ADMINISTERING SUBCUTANEOUS INJECTIONS OF FRAXIPARINE
FRAXIPARINE
- 1. You should wash your hands thoroughly with soap and water and dry them with a towel.
- 2. You should sit or lie down in a comfortable position. You should choose an injection site on the side of the abdomen (Figure 1). Injections should be administered alternately on the left and right sides.
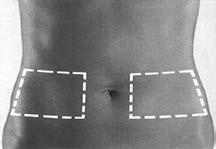
Figure 1
- 3. The injection site should be wiped with a swab soaked in spirit.
- 4. Remove the needle shield. The needle shield should be discarded.
Important notes
- You should not touch the needle and should not allow the needle to come into contact with other surfaces before injection.
- The presence of a small air bubble in the pre-filled syringe is normal. You should not remove the air bubble before administering the injection - this may lead to loss of part of the medicine.
- 5. You should gently grasp the previously cleaned skin with your fingers, creating a skin fold. The skin fold should be held between the thumb and index finger during the entire injection process (Figure 2).
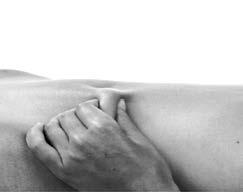
Figure 2
- 6. The pre-filled syringe should be held firmly by the fingers on the grip. The entire length of the needle should be inserted at a right angle into the skin fold (Figure 3).
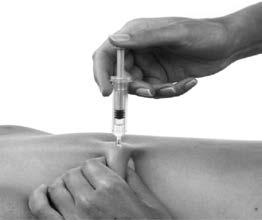
Figure 3
- 7.You should inject the entire contents of the pre-filled syringe by pressing the plunger down until resistance is felt.
- 8. Remove the needle with the pre-filled syringe from the skin (Figure 4). You should not rub the injection site.
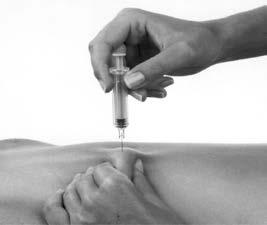
Figure 4
- 9. The appearance of the pre-filled syringe after injection (Figure 5).

Protective needle shield
Pre-filled syringe grip
Figure 5
- 10. After injection, you should slide the protective shield onto the pre-filled syringe to protect against needlestick injury (Figure 6). Holding the pre-filled syringe grip firmly with one hand, with the other hand you should grasp the outer shield of the pre-filled syringe and slide it towards the needle. The shield will unlock. Then you should slide the shield to the position where it will block the entire needle. During the release and locking of the shield, resistance will be felt.
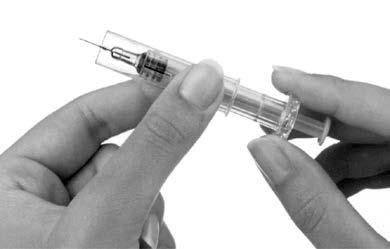
Figure 6
- 11. The appearance of the pre-filled syringe after sliding the protective shield (Figure 7).

Protective needle shield
Pre-filled syringe grip
Figure 7
- 12. The used pre-filled syringe should not be thrown into household waste containers. It should be disposed of in accordance with the doctor's or pharmacist's instructions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAspen Notre Dame de Bondeville GlaxoSmithKline Pharmaceuticals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FraxiparineDosage form: Solution, 5700 IU/0.6 mlActive substance: nadroparinPrescription requiredDosage form: Solution, 9500 IU aXa/mlActive substance: nadroparinPrescription requiredDosage form: Solution, 7600 IU aXa/0.8 mlActive substance: nadroparinPrescription required
Alternatives to Fraxiparine in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fraxiparine in Ukraine
Alternative to Fraxiparine in Spain
Online doctors for Fraxiparine
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fraxiparine – subject to medical assessment and local rules.














