

Fragmin

Ask a doctor about a prescription for Fragmin

How to use Fragmin
Leaflet accompanying the packaging: patient information
FRAGMIN
2,500 anti-Xa IU/0.2 mL, solution for injection
FRAGMIN
5,000 anti-Xa IU/0.2 mL, solution for injection
FRAGMIN
7,500 anti-Xa IU/0.3 mL, solution for injection
FRAGMIN
10,000 anti-Xa IU/0.4 mL, solution for injection
FRAGMIN
12,500 anti-Xa IU/0.5 mL, solution for injection
FRAGMIN
15,000 anti-Xa IU/0.6 mL, solution for injection
FRAGMIN
18,000 anti-Xa IU/0.72 mL, solution for injection
Dalteparinum natricum
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Fragmin and what is it used for
- 2. Important information before using Fragmin
- 3. How to use Fragmin
- 4. Possible side effects
- 5. How to store Fragmin
- 6. Contents of the packaging and other information
1. What is Fragmin and what is it used for
Fragmin is an anticoagulant medicine, in the form of dalteparin sodium. Dalteparin sodium is a low molecular weight heparin. The anticoagulant effect of dalteparin is related to its ability to enhance the inhibition of factor Xa and thrombin.
Fragmin is indicated for use in adult patients over 18 years of age for:
- Treatment of acute deep vein thrombosis.
- Unstable angina (e.g., rest angina, myocardial infarction without Q-wave).
- Chronic treatment of symptomatic venous thromboembolism (proximal deep vein thrombosis and pulmonary embolism) to reduce the risk of recurrent venous thromboembolism in patients with cancer.
- Prevention of blood clotting in extracorporeal circulation, e.g., during hemodialysis and hemofiltration in acute or chronic renal failure.
- Prophylactic anticoagulation related to surgical procedures.
- Prophylactic anticoagulation in medically immobilized patients: with congestive heart failure in class III or IV NYHA or acute respiratory failure, with acute infection, with acute rheumatic disease or acute inflammatory bowel disease and at least one additional risk factor for deep vein thrombosis, e.g., age over 75 years, obesity, cancer, history of deep vein thrombosis.
Fragmin is indicated for use in children for:
- Treatment of blood clots in the veins (venous thromboembolism (VTE)) in children and adolescents from 1 month of age and older.
2. Important information before using Fragmin
When not to use Fragmin
- If the patient is allergic to dalteparin sodium or other low molecular weight heparins, or unfractionated heparin, or any of the other ingredients of this medicine (listed in section 6);
- Confirmed or suspected heparin-induced thrombocytopenia of immunological origin;
- Active bleeding, e.g., from the gastrointestinal tract or cerebral vessels;
- Severe coagulation disorders;
- Acute or subacute bacterial endocarditis;
- Recent injuries or surgical procedures in the central nervous system, eyes, and (or) ears. Due to the increased risk of bleeding, high doses of dalteparin sodium (e.g., those used in the treatment of deep vein thrombosis, pulmonary embolism, or unstable angina) should not be used in patients who have recently undergone spinal anesthesia or epidural anesthesia, or other procedures that require lumbar puncture.
Warnings and precautions
Before starting treatment with Fragmin, you should discuss it with your doctor or pharmacist.
- In patients before spinal anesthesia (epidural or spinal) or lumbar puncture. Patients receiving such anticoagulant medications as low molecular weight heparins or heparinoids for the prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma, which can cause long-term or permanent paralysis. The risk of such complications is increased in cases where a permanent catheter is inserted into the epidural space for the administration of anesthetics, and in cases of concurrent use of medications that affect hemostasis, such as non-steroidal anti-inflammatory drugs, antiplatelet agents, and other anticoagulants. It appears that traumatic or repeated epidural or spinal puncture also increases this risk. Such patients should be monitored frequently, checking their condition for signs of neurological changes. In patients with: thrombocytopenia, platelet function disorders, severe liver or kidney dysfunction, uncontrolled or untreated hypertension, or hypertensive retinopathy or diabetic retinopathy.
- In patients for thromboprophylaxis, insertion or removal of an epidural catheter or spinal cord catheter should be performed at least 10-12 hours after administration of dalteparin. In patients receiving higher doses of Fragmin (e.g., 100-120 IU/kg body weight every 12 hours or 200 IU/kg body weight once daily), the time interval should be at least 24 hours.
- In patients who have undergone epidural or spinal anesthesia, it is necessary to ensure monitoring for signs of neurological failure, such as back pain, numbness or weakness of the lower limbs, bowel and bladder dysfunction. If these symptoms occur, you should immediately notify the nurse or doctor.
- In patients suspected of having epidural or spinal hematoma, treatment may require decompression of the spinal cord.
- In patients with prosthetic heart valves. Prophylactic doses of Fragmin are not sufficient to prevent valve thrombosis in these patients.
- In patients with pulmonary embolism who also have circulatory disorders, low blood pressure, or are in shock, due to the lack of clinical experience with the use of Fragmin.
- In patients with rapidly progressing or severe thrombocytopenia (platelet count less than 100,000/µL or mm^3) during treatment with Fragmin. The doctor should recommend platelet count testing before starting treatment with Fragmin and regular monitoring of this parameter during treatment. In any case, it is recommended to perform an in vitro test for anti-platelet antibodies in the presence of unfractionated heparin or low molecular weight heparins. If the result of this test is positive or unclear, or if such a test is not performed, the doctor should discontinue treatment with Fragmin.
- In pediatric patients, patients with renal failure, or patients with significant underweight or obesity, pregnant women, or individuals at increased risk of bleeding, or recurrence of thrombosis, in whom the doctor should consider monitoring the anticoagulant effect of dalteparin sodium. The doctor will prescribe the necessary tests.
- In patients undergoing long-term dialysis, the doctor will prescribe appropriate dose adjustment of Fragmin after performing tests of anti-Xa activity. In patients undergoing hemodialysis for acute indications, frequent monitoring of anti-Xa activity is necessary.
- In patients with unstable angina, i.e., unstable angina or myocardial infarction without Q-wave, in whom a full-size myocardial infarction has occurred. In these patients, the doctor may prescribe thrombolytic treatment (restoring blood flow in a closed or narrowed blood vessel). Concurrent use of Fragmin and thrombolytic medication increases the risk of bleeding, but this does not mean that Fragmin should be discontinued.
- In patients with unstable angina treated with Fragmin for a long time, in case of impaired renal function (creatinine concentration >150 µmol/L). In these patients, the doctor may consider reducing the dose.
- In patients with allergy or suspected allergy to latex (natural rubber), or in individuals who have come into contact with the needle shield of Fragmin in pre-filled syringes with confirmed or suspected latex allergy (natural rubber). The needle shield of Fragmin in pre-filled syringes may contain latex (natural rubber). In these individuals with hypersensitivity to latex, severe allergic reactions may occur. Due to the risk of hematoma, it is recommended to avoid performing any intramuscular injections in patients treated with Fragmin at a daily dose above 5,000 IU.
Interchangeability with other anticoagulant medications
Dalteparin cannot be used interchangeably (unit for unit) with unfractionated heparin, other low molecular weight heparins, or synthetic polysaccharides. Each of these medications differs in terms of the raw materials used, the manufacturing process, and physical, chemical, biological, and clinical properties, which leads to differences in dosing and possibly clinical efficacy and safety. Each of these medications is unique and requires adherence to individual medical recommendations for use.
Children
Fragmin is not used in newborns under 1 month of age.
Fragmin and other medications
You should tell your doctor or pharmacist about all medications you are currently taking or have recently taken, as well as any medications you plan to take.
Some medications may affect the action of Fragmin, and Fragmin may reduce the effectiveness of other medications taken at the same time.
Thrombolytic treatment (dissolving blood clots) or certain medications that affect blood clotting may increase the risk of bleeding when used concurrently with Fragmin:
- Aspirin (acetylsalicylic acid);
- Medications that inhibit platelet aggregation (used to reduce platelet aggregation and reduce the risk of blood clot formation);
- Thrombolytic medications (used to dissolve blood clots);
- Non-steroidal anti-inflammatory drugs (NSAIDs) (medications used to treat inflammatory conditions);
- GP IIb/IIIa receptor antagonists (medications that affect platelet aggregation, used to treat heart diseases);
- Vitamin K antagonists and other types of anticoagulant medications;
- Dextrans (used in some artificial tears).
Medications that increase the effect of Fragmin:
- Medications used to thin the blood (dipyridamole);
- Certain medications used to treat gout (e.g., sulfinpyrazone, probenecid);
- Certain diuretics (e.g., ethacrynic acid);
- Solutions administered to increase blood volume;
- Cytostatic medications (used to treat cancer).
Medications that may reduce the effect of Fragmin:
- Medications used to treat allergies and hay fever (e.g., antihistamines);
- Medications used to treat heart failure and cardiovascular diseases (e.g., medications containing digitalis, such as digoxin, digitoxin);
- Antibiotics from the tetracycline group, which are used to treat bacterial infections;
- Vitamin C (e.g., contained in vitamin preparations).
Other medications that may affect the action of Fragmin:
- Medications used to treat angina (e.g., intravenously administered nitroglycerin);
- Antibiotics, such as penicillins administered in high doses, which are used to treat bacterial infections;
- Medications used to prevent and treat malaria (e.g., quinine);
- Nicotine taken due to smoking or smoking cessation preparations.
It should be remembered that if the patient is being treated with Fragmin due to unstable angina, the doctor may adjust the dose of acetylsalicylic acid (aspirin).
Recommendations for dosing in children are based on clinical experience; data from clinical trials are too limited for the doctor to adjust the appropriate dose of Fragmin.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, suspects that she may be pregnant, or plans to have a child, she should consult her doctor or pharmacist before using this medication.
Pregnancy
The medication should be used during pregnancy only if it is absolutely necessary.
Breastfeeding
Small amounts of dalteparin sodium pass into human milk. The risk to breastfed children cannot be ruled out. Fragmin should be used during breastfeeding, considering the benefits of breastfeeding for the child and the benefits of therapy for the mother.
Fertility
There is no data indicating the effect of Fragmin on fertility.
Driving and using machines
Fragmin does not affect the ability to drive vehicles and operate machinery.
Fragmin contains sodium
Fragmin 2,500 anti-Xa IU/0.2 mL, Fragmin 5,000 anti-Xa IU/0.2 mL, Fragmin 7,500 anti-Xa IU/0.3 mL, Fragmin 10,000 anti-Xa IU/0.4 mL, Fragmin 12,500 anti-Xa IU/0.5 mL, Fragmin 15,000 anti-Xa IU/0.6 mL, and Fragmin 18,000 anti-Xa IU/0.72 mL contain less than 1 mmol (23 mg) of sodium per pre-filled syringe, which means that the medication is considered "sodium-free". Patients on a low-sodium diet and parents whose children are being treated with Fragmin can be informed that these medications are essentially "sodium-free".
This medication may be diluted with solutions containing sodium. You should inform your doctor if the patient or child is on a low-sodium diet.
3. How to use Fragmin
This medication should always be used in accordance with the doctor's or pharmacist's recommendations. In case of doubts, you should consult your doctor or pharmacist.
Fragmin is administered subcutaneously, intravenously, or extracorporeally (in the dialysis circuit).
Fragmin should not be administered intramuscularly.
In the treatment of blood clots, Fragmin is administered subcutaneously.
If the patient is undergoing hemodialysis or hemofiltration (processes used to purify the blood), Fragmin is administered intravenously or into the dialysis tube.
Dalteparin can be mixed with isotonic sodium chloride solution for infusion (9 mg/mL) or glucose (50 mg/mL) in glass bottles and plastic containers.
Dosing and administration Treatment of acute deep vein thrombosis
Fragmin can be administered subcutaneously in one or two doses per day.
During therapy with Fragmin, concurrent administration of vitamin K antagonists can be started.
- The treatment is continued until the prothrombin complex factors (factors II, VII, IX, X) decrease to a therapeutic level. This usually occurs after about 5 days of combined treatment. Ambulatory treatment with the same doses used in the hospital is possible.
Once-daily administration
The dose is 200 IU/kg body weight subcutaneously once daily. Monitoring of anticoagulant activity is not necessary. A single daily dose should not exceed 18,000 IU.
The dosing with a pre-filled syringe can be determined based on the table below.
| Body weight (kg) | Dose |
| 10,000 IU |
| 12,500 IU |
| 15,000 IU |
| 83 and more | 18,000 IU |
Twice-daily administration
Alternatively, a dose of 100 IU/kg body weight can be administered subcutaneously twice daily. Generally, monitoring of anticoagulant activity is not necessary, but it should be considered in special patients (see section 2: Warnings and precautions). Blood should be drawn when the maximum concentration of the medication in the plasma occurs (3 to 4 hours after subcutaneous injection). The recommended maximum plasma concentrations are between 0.5 and 1.0 anti-Xa IU/mL.
Prevention of blood clotting in extracorporeal circulation, e.g., during hemodialysis and hemofiltration in acute or chronic renal failure
- Patients with chronic renal failure and without increased bleeding riskIn these patients, usually only minor adjustments of the dose are necessary, so frequent monitoring of anti-Xa activity is not required. However, in most of them, monitoring is not necessary.
- Hemodialysis and hemofiltration lasting no longer than 4 hoursA single intravenous bolus injection can be administered at the beginning of the procedure. The recommended initial dose is 5,000 IU; a lower initial dose can be used if clinically indicated. The initial dose of 5,000 IU for the single bolus dose regimen can be adjusted from session to session based on the result of the previous dialysis; the dose can be increased or decreased gradually by 500 or 1,000 IU until a satisfactory effect is achieved. Alternatively, an intravenous bolus injection of 30 to 40 IU/kg body weight can be administered, followed by an intravenous infusion of 10 to 15 IU/kg body weight per hour.
- Hemodialysis and hemofiltration lasting more than 4 hoursA single intravenous bolus injection of 30 to 40 IU/kg body weight, followed by an intravenous infusion of 10 to 15 IU/kg body weight per hour.
- Patients with acute renal failure or increased bleeding riskIntravenous bolus injection of 5 to 10 IU/kg body weight, followed by intravenous infusion of 4 to 5 IU/kg body weight per hour. Such patients may be more unstable and may require monitoring of anti-Xa levels. The anti-Xa concentration in plasma should be between 0.2 and 0.4 IU/mL.
Prophylactic anticoagulation related to surgical procedures
Fragmin should be administered subcutaneously. Monitoring of anticoagulant activity is generally not necessary. If monitoring is performed, blood should be drawn when the maximum concentration of the medication in the plasma occurs (3 to 4 hours after subcutaneous injection). The recommended doses usually result in maximum plasma concentrations between 0.1 and 0.4 anti-Xa IU/mL.
General surgical procedures
You should choose the appropriate dosing regimen from the options listed below.
- Patients at risk of thromboembolic complications2,500 IU subcutaneously 2 hours before surgery and 2,500 IU subcutaneously every morning after surgery until the patient is mobilized (usually for 5 to 7 days or longer).
- Patients with additional risk factors for thromboembolic complications (e.g., cancer)Administer Fragmin until the patient is mobilized (usually for 5 to 7 days or longer).
- 1. Starting treatment the day before surgery: 5,000 IU subcutaneously in the evening before surgery. After surgery, 5,000 IU subcutaneously every evening.
- 2. Starting treatment on the day of surgery: 2,500 IU subcutaneously within 2 hours before surgery and 2,500 IU subcutaneously 8 to 12 hours later, but not earlier than 4 hours after the end of surgery. After surgery, starting from the next day, 5,000 IU subcutaneously every morning.
Orthopedic surgery (e.g., hip replacement surgery)
Administer Fragmin for up to 5 weeks after surgery, choosing one of the dosing regimens described below.
- 1. Starting treatment the evening before surgery: 5,000 IU subcutaneously in the evening before surgery. After surgery, 5,000 IU subcutaneously every evening.
- 2. Starting treatment on the day of surgery: 2,500 IU subcutaneously within 2 hours before surgery and 2,500 IU subcutaneously 8 to 12 hours later, but not earlier than 4 hours after the end of surgery. Starting from the day after surgery, 5,000 IU subcutaneously every morning.
- 3. Starting treatment after surgery: 2,500 IU subcutaneously 4 to 8 hours after surgery, but not earlier than 4 hours after the end of surgery. Starting from the day after surgery, 5,000 IU subcutaneously every morning.
Prophylaxis of acute deep vein thrombosis in medically immobilized patients
The recommended dose of Fragmin is 5,000 IU once daily. Treatment with dalteparin sodium is continued until the end of the patient's immobilization period, for 14 days or longer. Monitoring of anticoagulant activity is generally not necessary.
Duration of administration
Prophylactic anticoagulation in patients with significantly increased risk of thromboembolic complications, temporarily immobilized due to severe illness, such as heart failure, respiratory failure, or severe infection, should be continued until the patient is fully mobilized. The duration of use is determined based on the patient's condition and usually lasts 14 days.
Method of administration
Before injecting, the drop at the end of the needle should be removed, as the introduction of dalteparin sodium into the injection canal may lead to the formation of a harmless superficial hematoma or, in rare cases, local irritation.
To familiarize yourself with the detailed instructions for administration, see the section below How to inject Fragmin
Fragmin. Unstable angina (unstable angina and myocardial infarction without Q-wave)
Generally, monitoring of anticoagulant activity is not necessary, but it should be considered in special patients (see section 2: Warnings and precautions). Blood should be drawn when the maximum concentration of the medication in the plasma occurs (3 to 4 hours after subcutaneous injection). The recommended maximum plasma concentrations are between 0.5 and 1.0 anti-Xa IU/mL. It is recommended to administer acetylsalicylic acid (aspirin) concurrently (in a dose of 75 to 325 mg/day). Fragmin should be administered at a dose of 120 IU/kg body weight subcutaneously every 12 hours, up to a maximum dose of 10,000 IU/12 hours. Treatment should be continued until the patient's clinical condition stabilizes (usually for at least 6 days) or longer if the doctor considers it beneficial. Then, prolonged treatment with a fixed dose of Fragmin is recommended until revascularization procedures (such as percutaneous interventions or coronary artery bypass grafting) are performed. The medication should not be used for more than 45 days. The dose of Fragmin is determined based on the patient's sex and body weight:
- In the case of women with a body weight of less than 80 kg and men with a body weight of less than 70 kg, 5,000 IU should be administered subcutaneously every 12 hours.
- In the case of women with a body weight of 80 kg or more and men with a body weight of 70 kg or more, 7,500 IU should be administered subcutaneously every 12 hours.
Chronic treatment of symptomatic venous thromboembolism (proximal deep vein thrombosis and pulmonary embolism) to reduce the risk of recurrent venous thromboembolism in patients with cancer
Month 1
During the first 30 days of treatment, Fragmin should be administered once daily, subcutaneously (sc.), at a dose of 200 IU/kg body weight. The total daily dose should not exceed 18,000 IU.
Months 2-6
Fragmin should be administered once daily, subcutaneously, at a dose of approximately 150 IU/kg body weight. The dose of the medication administered from a single-dose pre-filled syringe is determined based on the table below.
Reducing the dose in case of chemotherapy-induced thrombocytopenia
The following dosing rules were used in clinical trials:
In case of chemotherapy-induced thrombocytopenia with a platelet count of less than 50,000/mm^3, Fragmin administration should be discontinued until the platelet count returns to above 50,000/mm^3. If the platelet count is between 50,000 and 100,000/mm^3, the dose of Fragmin should be reduced by 17% to 33% of the initial dose (depending on the patient's body weight). Once the platelet count returns to 100,000/mm^3 or more, the full dose of Fragmin should be resumed.
Table 1. Reduction of Fragmin dose in case of thrombocytopenia in the range of 50,000 – 100,000/mm^3, dosing using single-dose pre-filled syringes
Kidney dysfunction
In case of significant kidney dysfunction defined as creatinine concentration exceeding three times the upper limit of normal, the dose of Fragmin should be adjusted to maintain a therapeutic anti-Xa concentration of 1 IU/mL (range 0.5-1.5 IU/mL), measured 4-6 hours after injection. If the anti-Xa concentration is below or above the therapeutic range, the dose of Fragmin should be appropriately increased or decreased. The measurement of anti-Xa concentration should be repeated after administration of 3-4 injections at the newly adjusted dose. This dose adjustment scheme should be repeated until anti-Xa reaches a therapeutic level.
| Body weight (kg) | Fragmin dose (IU) |
| ≤56 | 7,500 |
| 10,000 |
| 12,500 |
| 15,000 |
| ≥99 | 18,000 |
| Body weight (kg) | Planned Fragmin dose (IU) | Reduced Fragmin dose (IU) | Average dose reduction (%) |
| ≤56 | 7,500 | 5,000 | 33 |
| 10,000 | 7,500 | 25 |
| 12,500 | 10,000 | 20 |
| 15,000 | 12,500 | 17 |
| ≥99 | 18,000 | 15,000 | 17 |
Use in children and adolescents
Treatment of blood clots in the veins (venous thromboembolism (VTE))
The recommended doses depend on the child's body weight and age group, and will be calculated by the doctor.
The doctor will inform you about the individualized dose of Fragmin according to these criteria. You should not change the dosing regimen without consulting the doctor.
The recommended initial dose for children and adolescents is as follows:
Children from 1 month to less than 2 years of age:150 IU/kg body weight twice daily.
Children from 2 to less than 8 years of age:125 IU/kg body weight twice daily.
Children from 8 to less than 18 years of age:100 IU/kg body weight twice daily.
The effect of Fragmin is monitored after administration of the initial dose, and then the dose is adjusted based on blood tests.
How to inject Fragmin
Fragmin is administered subcutaneously. This part of the leaflet explains how to administer Fragmin to yourself or your child. You should follow these instructions only after training by a doctor. If you are unsure what to do, you should immediately contact your doctor.
You should inject (administer) the dose of Fragmin at the times of the day recommended by your doctor.
If dilution is required before administration of Fragmin to children, it should be performed by medical personnel. You should follow the doctor's recommendations regarding the method and time of administration of the diluted medication.
You should perform the steps described below
Step 1: Preparing the pre-filled syringe for injection of Fragmin.
You should remove the cap from the pre-filled syringe. A air bubble will appear in the pre-filled syringe. It should be there and ignored. It is important not to press the plunger yet, as this may cause loss of the medication, and thus a reduction in the dose.
The pre-filled syringe is ready for injection. You should proceed to step 2.
Pre-filled syringe with Needle-Trap needle protection
The Needle-Trap needle protection system has been specially designed to prevent needle stick injuries after proper administration of Fragmin. It consists of a plastic protective device attached to the label on the syringe. It is used to avoid accidental needle sticks after proper injection of Fragmin. The Needle-Trap needle protection system consists of a plastic latch (handle) placed parallel to the needle, firmly attached to the label on the syringe cylinder.
To activate the protection system, you should perform the following steps: Lift the syringe, grasp the end of the plastic needle handle, and bend it away from the needle shield (see Fig. 1).
Fig. 1
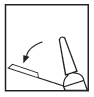
You should remove the gray rubber needle shield by pulling it straight off (see Fig. 2).
Fig. 2
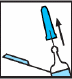
A air bubble can be seen in the pre-filled syringe. It should be there and ignored. It is important not to press the plunger yet, as this may cause loss of the medication, and thus a reduction in the dose. Air bubbles in single-dose pre-filled syringes should not be expelled before injection, as this may cause loss of the medication, and thus a reduction in the dose.
The pre-filled syringe is ready for injection. You should proceed to step 2.
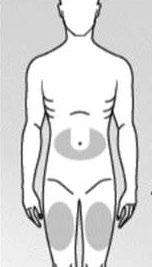
Step 2: Choosing and preparing the injection site
You should choose one of the recommended injection sites below (see shaded areas in Fig. 3):
A "U"-shaped area around the navel.
The middle of the thigh.
Fig. 3
- Each time you administer a dose, you should inject it into a different site.
- Do not inject into areas where the skin is sensitive, bruised, red, or hard. You should avoid areas with scars.
- If the adult or child has psoriasis, the medication should not be injected directly into any raised, thick, red, or scaly skin patches ("psoriatic skin lesions").
- You should wash and dry your hands.
- You should clean the injection site with a new alcohol swab, moving in a circular motion. The skin should dry completely. You should not touch this area again before performing the injection.
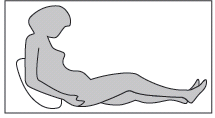
Step 3: Assuming the correct position
The adult or child should sit or lie down for deep subcutaneous injection. If the patient is performing the injection themselves, they should sit in a comfortable position, so that they can see their stomach (see Fig. 4).
Fig. 4
Step 4:
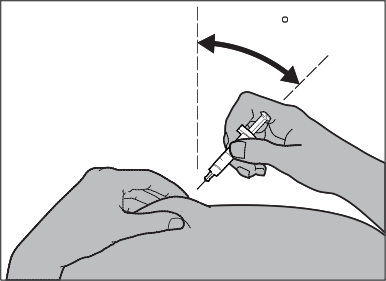
Using your thumb and index finger, you should grasp a fold of skin with one hand. With the other hand, you should hold the syringe like a pencil. This will be the injection site.
Step 5:
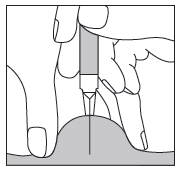
In the case of injecting Fragmin to an adult or yourself, you should hold the pre-filled syringe over the skin fold at a right angle (i.e., vertically, as shown, and not at an angle). You should insert the needle into the skin until it is fully inserted (see Fig. 5).
Fig. 5
In the case of injecting Fragmin to a child, you should insert the needle into the skin with a quick, short motion at an angle of 45° to 90° (see Fig. 6).
Fig. 6
Step 6:
You should press the plunger all the way down at a slow, steady pace to administer the correct dose.
While injecting, you should continue to hold the skin fold, and then release it and withdraw the needle.
If bleeding occurs at the injection site, you should gently press on it. You should not rub the injection site, as this may cause bruising.
You should apply a swab to the injection site for 10 seconds. Minor bleeding may occur. You should not rub the injection site. The injection site can be covered with a bandage.
| Step 6: If the syringe has a Needle-Trap needle protection system | |
| needle protection | |
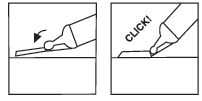
| You should place the plastic handle on a hard, stable surface and, with one hand, turn the syringe cylinder upwards towards the needle, pushing the needle into the sheath, where it will lock into place (see Fig. 7 | |
| You should continue bending the needle until the syringe exceeds an angle of 45 degrees with the flat surface, making it permanently unusable (see Fig. 8 | |
Fig. 7 Fig. 8
Step 7:
You should dispose of the pre-filled syringe and needle in a sharps container. You should keep the sharps container in a place inaccessible to others. When the sharps container is almost full, you should dispose of it according to the instructions or contact your doctor or nurse.
The dose will be adjusted based on the child's age and body weight. In younger children, a slightly higher dose of Fragmin may be required per kilogram of body weight than in adults. The doctor will adjust the appropriate dose for the patient. To monitor the effect of Fragmin, during treatment, medical personnel may draw a blood sample.
Use of Fragmin in patients with kidney and liver dysfunction
The medication requires cautious use in patients with kidney and liver dysfunction (see section 2: Warnings and precautions).
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Side effects are listed according to their frequency.
Frequent(may occur in no more than 1 in 10 people)
- mild, reversible thrombocytopenia (type I)
- bleeding
- transient increase in liver enzyme activity (aminotransferases, AspAT, AlAT)
- subcutaneous hematoma at the injection site
- pain at the injection site
Infrequent(may occur in no more than 1 in 100 people)
- hypersensitivity
Rare(may occur in no more than 1 in 1,000 people)
- skin necrosis
- transient alopecia
Frequency not known(cannot be estimated from the available data)
- heparin-induced immunological thrombocytopenia (type II, with or without thrombotic complications)
- anaphylactic reactions
- intracranial bleeding (some fatal)
- bleeding into the retroperitoneal space (some fatal)
- rash
- subarachnoid or epidural hematoma
Heparin products can cause hypoaldosteronism (decreased aldosterone secretion - a hormone of the adrenal cortex), which can lead to increased potassium levels in the blood (hyperkalemia). Rarely, especially in patients with chronic renal failure and diabetes, clinically significant hyperkalemia may occur. With long-term use of Fragmin, there is a risk of osteoporosis. It is estimated that the side effects in children will be the same as in adults, but there is only limited data on the occurrence of possible side effects during long-term treatment in children.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the representative of the marketing authorization holder or the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Fragmin
The medicine should be stored out of sight and reach of children. Prefilled syringes should be stored at a temperature below 25°C. Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month. From a microbiological point of view, the product should be used immediately. If it is not used immediately, the user is responsible for the storage time and conditions during use. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and other information
What Fragmin contains
- The active substance is dalteparin sodium. The potency is expressed in anti-Xa international units (IU) in accordance with the First International Standard for Low Molecular Weight Heparins.
- The other ingredients are:
What Fragmin looks like and what the pack contains
The solution for injection is available in a prefilled syringe with a needle containing a single dose of the medicine (Type I glass) with a needle shield (rubber), plunger stopper (chlorobutyl rubber), and plunger (polypropylene or polystyrene) with or without a Needle-Trap as a safety device. The needle shield may contain latex.
| Nr | Volume | Dalteparin sodium (Dalteparinum natricum) |
| 0.2 ml | 2,500 anti-Xa IU |
| 0.2 ml | 5,000 anti-Xa IU |
| 0.3 ml | 7,500 anti-Xa IU |
| 0.4 ml | 10,000 anti-Xa IU |
| 0.5 ml | 12,500 anti-Xa IU |
| 0.6 ml | 15,000 anti-Xa IU |
| 0.72 ml | 18,000 anti-Xa IU |
| Nr | Dose/Volume | Excipients |
| 2,500 anti-Xa IU/0.2 ml | sodium chloride, sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
| 5,000 anti-Xa IU/0.2 ml | sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
| 7,500 anti-Xa IU/0.3 ml | sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
| 10,000 anti-Xa IU/0.4 ml | sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
| 12,500 anti-Xa IU/0.5 ml | sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
| 15,000 anti-Xa IU/0.6 ml | sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
| 18,000 anti-Xa IU/0.72 ml | sodium hydroxide or hydrochloric acid (pH 5-7.5 adjustment), water for injections |
Not all pack sizes may be marketed.
Marketing authorization holder
Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Brussels, Belgium
Manufacturer
2,500 anti-Xa IU/0.2 ml; 5,000 anti-Xa IU/0.2 ml; 7,500 anti-Xa IU/0.3 ml: Pfizer Manufacturing Belgium NV, Rijksweg 12, 2870 Puurs-Sint-Amands, Belgium; Catalent France Limoges S.A.S., Z.I. Nord, 53 rue de Dion Bouton, 87280 Limoges, France. 10,000 anti-Xa IU/0.4 ml, 12,500 anti-Xa IU/0.5 ml; 15,000 anti-Xa IU/0.6 ml; 18,000 anti-Xa IU/0.72 ml: Pfizer Manufacturing Belgium NV, Rijksweg 12, 2870 Puurs-Sint-Amands, Belgium. For more detailed information on this medicine, please contact the local representative of the marketing authorization holder: Pfizer Polska Sp. z o.o., tel. 22 335 61 00.
Date of last revision of the leaflet: 06/2025
Detailed and up-to-date information about this product can be obtained by scanning the QR code on the outer packaging using a mobile device. The same information is also available at the URL: https://pfi.sr/ulotka-fragmin and on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: http://www.urpl.gov.pl.
| Nr | Dose/Volume | Type and content of packaging |
| 2,500 anti-Xa IU/0.2 ml | 10 prefilled syringes with a needle, 0.2 ml each; 20 prefilled syringes with a needle, 0.2 ml each |
| 5,000 anti-Xa IU/0.2 ml | 10 prefilled syringes with a needle, 0.2 ml each; 20 prefilled syringes with a needle, 0.2 ml each |
| 7,500 anti-Xa IU/0.3 ml | 10 prefilled syringes with a needle, 0.3 ml each; 20 prefilled syringes with a needle, 0.3 ml each |
| 10,000 anti-Xa IU/0.4 ml | 5 prefilled syringes with a needle, 0.4 ml each |
| 12,500 anti-Xa IU/0.5 ml | 5 prefilled syringes with a needle, 0.5 ml each |
| 15,000 anti-Xa IU/0.6 ml | 5 prefilled syringes with a needle, 0.6 ml each |
| 18,000 anti-Xa IU/0.72 ml | 5 prefilled syringes with a needle, 0.72 ml each |
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterCatalent France Limoges S.A.S Z.I. Nord Pfizer Manufacturing Belgium NV
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FragminDosage form: Solution, 2500 IU anti-Xa/0.2 mlActive substance: dalteparinPrescription requiredDosage form: Solution, 5000 IU anti-Xa/0.2 mlActive substance: dalteparinPrescription requiredDosage form: Solution, 7500 IU anti-Xa/0.3 mlActive substance: dalteparinPrescription required
Alternatives to Fragmin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fragmin in Hiszpania
Online doctors for Fragmin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fragmin – subject to medical assessment and local rules.














