
Fostex Nexthaler

Ask a doctor about a prescription for Fostex Nexthaler

How to use Fostex Nexthaler
Leaflet accompanying the packaging: patient information
Fostex NEXThaler
(200 micrograms + 12 micrograms)/inhalation dose, inhalation powder
Beclometasone dipropionate + Formoterol fumarate dihydrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Fostex NEXThaler and what is it used for
- 2. Important information before using Fostex NEXThaler
- 3. How to use Fostex NEXThaler
- 4. Possible side effects
- 5. How to store Fostex NEXThaler
- 6. Package contents and other information
1. What is Fostex NEXThaler and what is it used for
Fostex NEXThaler is a powder that is inhaled through the mouth and reaches deep into the lungs.
The medicine contains two active substances: beclometasone dipropionate and formoterol fumarate dihydrate.
- Beclometasone dipropionate belongs to a group of medicines called corticosteroids, which have an anti-inflammatory effect by reducing swelling and irritation of the lungs.
- Formoterol fumarate dihydrate belongs to a group of medicines known as long-acting bronchodilators. They relax the muscles in the airways, which helps to widen the airways and make it easier to breathe in and out.
Together, these two active substances make it easier to breathe by relieving symptoms such as:
shortness of breath, wheezing, and coughing in patients with asthma or chronic obstructive pulmonary disease (COPD), and also help prevent asthma symptoms.
Asthma
Fostex NEXThaler is intended for the treatment of asthma in adults.
Fostex NEXThaler is used in patients whose:
- asthma is not adequately controlled with inhaled corticosteroids and as-needed short-acting bronchodilators or
- have achieved an adequate response to asthma treatment with both corticosteroids and long-acting bronchodilators.
1/11
DE/H/0871/006/DC
COPD
Fostex NEXThaler may also be used to treat symptoms of severe chronic obstructive pulmonary disease (COPD) in adult patients. COPD is a chronic respiratory disease in the lungs that is mainly caused by smoking.
2. Important information before using Fostex NEXThaler
When not to use Fostex NEXThaler:
Warnings and precautions
The medicine should not be used if the patient has acute asthma symptoms, such as shortness of breath, wheezing, and coughing, in case of asthma exacerbation or in case of an acute asthma attack. To relieve symptoms, a quick-acting inhaled reliever medicine should be used, which the patient should always carry with them.
Before starting Fostex NEXThaler, the patient should discuss with their doctor:
Before using Fostex NEXThaler, the patient should always inform their doctor if any of the above warnings apply to them.
If the patient is unsure whether Fostex NEXThaler can be used, they should consult their doctor, nurse, or pharmacist before using the medicine.
The doctor may recommend regular monitoring of potassium levels in the blood, especially in patients with severe asthma. Like many bronchodilators, Fostex NEXThaler may cause a sudden drop in potassium levels in the blood (hypokalemia).
Low oxygen levels in the blood in combination with certain medicines that the patient may be taking at the same time with Fostex NEXThaler may cause a further decrease in potassium levels in the blood.
If the patient takes high doses of inhaled corticosteroids for a long time, they may be more likely to need corticosteroids in stressful situations. Stressful situations may include: hospitalization after an accident, serious injury, or awaiting surgery. In such cases, the doctor will decide whether to increase the dose of corticosteroids or prescribe other steroids in the form of tablets or injections.
If hospitalization is necessary, the patient should remember to bring all their medicines and inhalers, including Fostex NEXThaler, as well as over-the-counter medicines, if possible, in their original packaging.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Children and adolescents
This medicine should not be given to children and adolescents under 18 years of age.
Fostex NEXThaler and other medicines
The patient should tell their doctor or pharmacist about all the medicines they are currently taking or have recently taken, as well as any medicines they plan to take. This is necessary because Fostex NEXThaler may affect the action of some other medicines. Similarly, some medicines may affect the action of Fostex NEXThaler.
In particular, the patient should tell their doctor or pharmacist if they are taking the following medicines:
- certain medicines may enhance the effect of Fostex NEXThaler and the doctor may want to closely monitor the patient's condition when taking such medicines (including certain HIV medicines: ritonavir, cobicistat);
- beta-blockers. Beta-blockers are medicines used to treat many diseases, including heart disorders, high blood pressure, or glaucoma (increased pressure in the eye). If beta-blockers (including eye drops) need to be administered, the effect of formoterol may be reduced or formoterol may not work at all;
- beta-adrenergic medicines (medicines that work in the same way as formoterol), which may enhance the effect of formoterol;
- medicines used to treat irregular heartbeat (quinidine, disopyramide, procainamide);
- medicines used to treat allergies (antihistamines such as terfenadine);
- medicines used to treat symptoms of depression or mental disorders, such as monoamine oxidase inhibitors (e.g., phenelzine and isocarboxazid) or tricyclic antidepressants (e.g., amitriptyline and imipramine) or phenothiazines;
- medicines used to treat Parkinson's disease (L-dopa);
- medicines used to treat hypothyroidism (L-thyroxine);
- medicines containing oxytocin (which cause uterine contractions);
- medicines used to treat mental disorders, such as monoamine oxidase inhibitors (MAOIs) or medicines with similar properties, such as furazolidone and procarbazine;
- medicines used to treat heart disease (digoxin);
- other medicines used to treat asthma (theophylline, aminophylline, or steroids);
- diuretics.
The patient should also inform their doctor if they are scheduled to undergo general anesthesia for surgery or a dental procedure.
Pregnancy, breastfeeding, and fertility
There are no clinical data on the use of Fostex NEXThaler during pregnancy.
Fostex NEXThaler should not be used if the patient is pregnant, thinks they may be pregnant, or plans to have a baby, or if the patient is breastfeeding, unless the doctor decides otherwise.
3/11
DE/H/0871/006/DC
Driving and using machines
It is unlikely that Fostex NEXThaler will affect the ability to drive and use machines. However, if the patient experiences side effects such as dizziness and/or tremors, they may affect the ability to drive and use machines.
Fostex NEXThaler contains lactose
Lactose contains small amounts of milk proteins, which may cause allergic reactions in patients.
3. How to use Fostex NEXThaler
This medicine should always be used as directed by the doctor. If the patient is unsure, they should consult their doctor or pharmacist.
Fostex NEXThaler uses extrafine particle technology, which delivers a larger portion of each dose to the lungs.
Therefore, the doctor may prescribe a lower dose of this medicine than the doses of inhaled medicine the patient has used before.
Asthma
The doctor will regularly check that the patient is taking the optimal dose of Fostex NEXThaler. Once adequate control of asthma is achieved, the doctor may consider gradually reducing the dose of Fostex NEXThaler. The dose should not be changed without prior consultation with the doctor.
Adults and the elderly:
The recommended dose is 1 inhalation twice a day.
The maximum daily dose is 2 inhalations.
Fostex NEXThaler should not be used to treat sudden worsening of asthma symptoms such as shortness of breath, wheezing, and coughing.
Note: The patient should always carry a quick-acting inhaled reliever medicine with them to relieve symptoms of asthma exacerbation or acute asthma attack.
The dose of Fostex NEXThaler should not be increased.
If the patient feels that the effect of the medicine is insufficient, they should consult their doctor before increasing the dose.
Chronic obstructive pulmonary disease (COPD)
Adults and the elderly:
The recommended dose is 1 inhalation twice a day.
The maximum daily dose is 2 inhalations.
How to use Fostex NEXThaler:
Fostex NEXThaler is intended for inhalation use.
If possible, the patient should stand or sit upright while inhaling.
If breathing difficulties do not improve:If the symptoms do not decrease after inhaling Fostex NEXThaler, the cause may be incorrect use of the inhaler. Therefore, the patient should check the instructions for correct use of the inhaler, which are below, and/or consult their doctor or nurse, who will re-instruct them on how to use the inhaler correctly.
4/11
DE/H/0871/006/DC
In case of worsening asthma symptoms:If the symptoms of the disease worsen or become more difficult to control (e.g., more frequent use of another inhaled medicine) or if the symptoms do not improve with the use of a reliever medicine, the patient should continue to use Fostex NEXThaler and consult their doctor immediately. The doctor may decide to change the dose of Fostex NEXThaler or use additional or alternative treatment.
INSTRUCTIONS FOR USING THE NEXTHALER INHALER
A. Package contents
To check the package contents, see section 6.
If the package contents differ from the description in section 6, the inhaler should be returned to the person from whom it was purchased and exchanged for a new one.
B. General warnings and precautions
- The inhaler should not be removed from the protective sachet unless it is to be used immediately.
- The inhaler should be used as directed.
- The cap should not be opened unless it is necessary to take the next dose using the inhaler.
- If the inhaler is not in use, it should be stored in a clean and dry place.
- Under no circumstances should the inhaler be disassembled into parts.
C. Main features of the inhaler
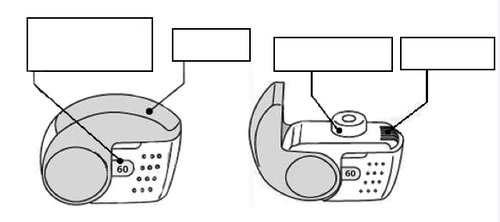
Dose counter window
Cap
Mouthpiece
Air inlet
To take a dose using the inhaler, the patient should perform three simple steps: open, inhale, and close.
D. Before using a new inhaler
- 1. Open the sachet and remove the inhaler. o Do not use the inhaler if the sachet is not sealed or is damaged
- the inhaler should be returned to the person from whom it was purchased and exchanged for a new one. o On the label attached to the box, the patient should write the date of opening the sachet.
- 2. Check the inhaler. o If the inhaler appears to be damaged or broken, it should be returned to the person from whom it was purchased and exchanged for a new one.
- 3. Check the dose counter window. If the inhaler is new, the dose counter will show "60".
5/11
DE/H/0871/006/DC
o The patient should not use a new inhaler if the dose counter window shows a number less than "60". The inhaler should be returned to the person from whom it was purchased and exchanged for a new one.
E. How to use the inhaler
- If the patient is unsure whether they have taken the correct dose, they should consult their doctor or pharmacist.
- If the patient is unsure whether the dose counter shows one less inhalation after use, they should wait until the next scheduled inhalation and take the medicine as usual. They should not take an extra dose.
E.1. Opening
- 1. Hold the inhaler firmly in a vertical position.
- 2. Check the number of doses remaining: any number between "1" and "60" indicates that there are still doses of medicine left in the inhaler. o If the dose counter shows "0", it means that there are no more doses left – the inhaler should be discarded and a new one purchased.
- 3. Open the inhaler cap completely.
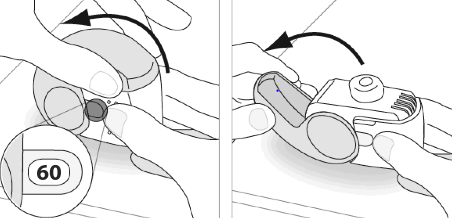
- 4. Before inhaling, the patient should exhale as deeply as possible. o The patient should not exhale through the inhaler.
E.2. Inhaling
If possible, the patient should stand or sit upright while inhaling.
- 1. Lift the inhaler, place it in the mouth, and hold the mouthpiece with the lips. o The patient should not block the air inlet. o The patient should not inhale through the air inlet.
- 2. Take a strong and deep breath in through the mouth. o During inhalation, the patient may feel a characteristic taste. o During inhalation, the patient may hear and feel a click. o The patient should not inhale through the nose. o The patient should not remove the inhaler from the mouth during inhalation.
6/11
DE/H/0871/006/DC
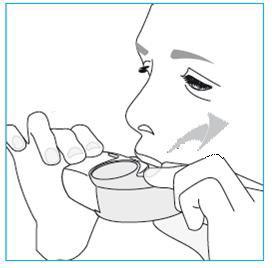
- 3. Remove the inhaler from the mouth.
- 4. Hold the breath for 5 to 10 seconds or as long as possible.
- 5. Exhale slowly. o The patient should not exhale through the inhaler.
E.3. Closing
- 1.Hold the inhaler in a vertical position and close the cap completely.
- 2.Check that the dose counter shows one less dose.
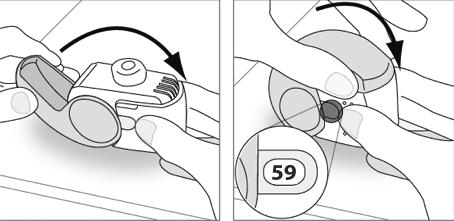
- 3.If an additional dose is required, the patient should repeat steps E.1 to E.3.
F. Cleaning
- Normally, cleaning the inhaler is not necessary.
- If necessary, the inhaler can be wiped with a dry cloth or tissue after use. o The inhaler should not be cleaned with water or other liquids. Store in a dry place.
G. Storage and disposal
For information on storing and disposing of the inhaler, see section 5.
Taking a higher dose of Fostex NEXThaler than recommended:
- the patient should contact their doctor or go to the emergency room of the nearest hospital for advice. The patient should bring the medicine with them so that healthcare professionals can see exactly what they have taken;
- side effects may occur. The patient should inform their doctor about any unusual symptoms, as they may recommend additional tests or take necessary measures.
Missing a dose of Fostex NEXThaler:
The patient should take the missed dose as soon as possible. If it is almost time for the next scheduled dose, the patient should not take the missed dose, but take the next dose at the usual scheduled time. The patient should not take a double dose.
7/11
DE/H/0871/006/DC
Stopping Fostex NEXThaler:
Even if the patient's condition improves, they should not stop using Fostex NEXThaler or reduce the dose without consulting their doctor. It is very important to regularly use Fostex NEXThaler as directed by the doctor, even when the symptoms of the disease disappear.
If the patient has any further doubts about using the medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Fostex NEXThaler can cause side effects, although not everybody gets them.
As with other inhaled medicines, there is a risk of worsening breathing difficulties, coughing, and wheezing immediately after taking Fostex NEXThaler, known as paradoxical bronchospasm. In this case, the patient should stop using Fostex NEXThaler immediately and use a quick-acting inhaled bronchodilator to relieve symptoms. The patient should consult their doctor immediately.
The patient should tell their doctor immediately if they experience any allergic reactions, such as:skin allergies, itching, skin rash, skin redness, skin swelling, or swelling of the skin and mucous membranes, especially of the eyes, face, lips, and throat.
Other possible side effects are listed below by frequency of occurrence.
Common (occurring in less than 1 in 10 people):
- tremors;
- pneumonia (lung infection) in patients with COPD
The patient should tell their doctor if they experience any of the following symptoms while using Fostex NEXThaler; these may be symptoms of a lung infection:
- fever or chills
- increased production of mucus or change in mucus color
- worsening cough or increased breathing difficulties
Uncommon (occurring in less than 1 in 100 people):
- flu-like symptoms, sore throat;
- fungal infections (of the mouth and throat). Rinsing the mouth and throat with water and brushing teeth immediately after inhalation may help prevent these side effects;
- worsening asthma symptoms, breathing difficulties;
- hoarseness;
- cough;
- rapid or irregular heartbeat;
- slow heartbeat;
- chest pain;
- headache;
- nausea;
- feeling tired or nervous;
- changes in electrocardiogram (ECG);
- low cortisol levels in urine or blood;
- high potassium levels in the blood;
- high glucose levels in the blood;
- high lipid levels in the blood.
8/11
DE/H/0871/006/DC
Rare (frequency cannot be estimated from available data):
- vision disturbances.
Side effects that have been observed with similar inhaled medicines containing beclometasone dipropionate and/or formoterol:
include:
- palpitations;
- irregular heartbeat;
- taste disturbances;
- muscle pain and cramps;
- nervousness, dizziness;
- restlessness;
- sleep disturbances;
- low potassium levels in the blood;
- increased or decreased blood pressure.
Long-term use of high doses of inhaled corticosteroids may
cause systemic effects.These include:
- adrenal gland disorders;
- bone thinning;
- growth retardation in children and adolescents;
- increased eye pressure (glaucoma), cataracts;
- rapid weight gain, especially in the face and chest area;
- sleep disturbances, depression or anxiety, nervousness, tension, overexcitement, or irritability. These side effects are more likely to occur in children;
- behavioral changes.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Fostex NEXThaler
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiration date stated on the carton, sachet, and inhaler label after "EXP". The expiration date refers to the last day of the month stated.
Store in the original packaging to protect from moisture. The inhaler should be removed from the sachet immediately before its first use.
9/11
DE/H/0871/006/DC
Before opening the sachet:There are no special storage temperature recommendations for the medicine.
After opening the sachet:Do not store above 25°C.
After opening the protective sachet, the shelf life of the medicine is 6 months.
The patient should write the date of opening the sachet on the label attached to the carton.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Fostex NEXThaler contains
The active substances of the medicine are beclometasone dipropionate and formoterol fumarate dihydrate.
Each dose (20 mg of inhalation powder) contains 200 micrograms of beclometasone dipropionate and 12 micrograms of formoterol fumarate dihydrate. This corresponds to a delivered dose from the mouthpiece containing 173.9 micrograms of beclometasone dipropionate and 10.4 micrograms of formoterol fumarate dihydrate.
The other ingredients of the medicine are lactose monohydrate (containing small amounts of milk proteins) and magnesium stearate.
What Fostex NEXThaler looks like and contents of the pack
The medicine is a white or almost white powder for inhalation in a plastic inhaler called Nexthaler.
One pack contains 1, 2, or 3 inhalers, each containing 60 doses of the medicine.
Each inhaler with an ABS closure and PP cap is in a PET/Aluminum/PE or Polyamide/Aluminum/PE bag. The whole thing is in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder:
Chiesi Farmaceutici S.p.A., Via Palermo 26/A, 43122 Parma, Italy
Manufacturer responsible for batch release:
Chiesi Farmaceutici S.p.A., Via San Leonardo 96, 43122 Parma, Italy
Chiesi S.A.S, 2 rue des Docteurs Alberto et Paolo Chiesi, 41260 La Chaussée Saint Victor, France
Chiesi Pharmaceuticals GmbH, Gonzagagasse 16/16, Innere Stadt, 1010 Vienna, Austria
To obtain more detailed information, the patient should contact the representative of the marketing authorization holder in Poland:
Chiesi Poland Sp. z o.o.,
Jerozolimskie Avenue 134, 02-305 Warsaw,
phone: (22) 620 14 21, fax: (22) 652 37 79, e-mail: [email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
10/11
DE/H/0871/006/DC
| Austria: | Foster NEXThaler | Netherlands: | Foster NEXThaler |
| France: | Innovair NEXThaler | Poland: | Fostex NEXThaler |
| Czech Republic: | Combair Nexthaler | Slovakia: | Foster NEXThaler |
| Germany: | Kantos NEXThaler 200 micrograms/12 micrograms per inhalation powder for inhalation | Slovenia: | Foster NEXThaler 200 micrograms/12 micrograms per inhalation, powder for inhalation |
| Greece: | Foster NEXThaler | Spain: | Foster NEXThaler |
| Hungary: | Foster NEXThaler | United Kingdom: | Fostair NEXThaler |
| Italy: | Foster |
Date of last revision of the leaflet:
{Chiesi logo}
11/11
DE/H/0871/006/DC
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterChiesi Farmaceutici S.p.A. Chiesi Pharmaceuticals GmbH Chiesi S.A.S.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Fostex NexthalerDosage form: Aerosol, (100 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription requiredDosage form: Aerosol, (200 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription requiredDosage form: Aerosol, (100 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription not required
Alternatives to Fostex Nexthaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fostex Nexthaler in Spain
Alternative to Fostex Nexthaler in Ukraine
Online doctors for Fostex Nexthaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fostex Nexthaler – subject to medical assessment and local rules.














