
Aerox
Ask a doctor about a prescription for Aerox

How to use Aerox
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: patient information
Aerox, (100 micrograms + 6 micrograms)/dose, inhalation aerosol, solution
Beclometasone dipropionate + Formoterol fumarate dihydrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Aerox and what is it used for
- 2. Important information before using Aerox
- 3. How to use Aerox
- 4. Possible side effects
- 5. How to store Aerox
- 6. Package contents and other information
1. What is Aerox and what is it used for
Aerox is a solution in an inhalation aerosol, containing two active substances that are inhaled directly into the lungs through the mouthpiece of the inhaler.
The medicine contains two active substances: beclometasone dipropionate and formoterol fumarate dihydrate.
Beclometasone dipropionate belongs to a group of medicines called corticosteroids, which have an anti-inflammatory effect by reducing swelling and irritation of the lungs.
Formoterol fumarate dihydrate belongs to a group of medicines called long-acting bronchodilators, which relax the muscles in the airways and make breathing easier.
Together, these two active substances make breathing easier by relieving symptoms such as:
shortness of breath, wheezing, and cough in patients with asthma or chronic obstructive pulmonary disease (COPD), and also help prevent asthma symptoms.
Asthma
Aerox is indicated for the regular treatment of asthma in adult patients, in whom:
- asthma symptoms are not adequately controlled with inhaled corticosteroids and a short-acting bronchodilator used as needed, or
- adequate control of asthma symptoms has been achieved with both inhaled corticosteroids and long-acting bronchodilators.
COPD
Aerox may also be used to treat symptoms of severe chronic obstructive pulmonary disease (COPD) in adult patients. COPD is a chronic respiratory disease in the lungs, mainly caused by smoking.
Aerox is intended for use in adults.
2. Important information before using Aerox
When not to use Aerox:
- if the patient is allergic to beclometasone dipropionate, formoterol fumarate dihydrate, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Aerox, discuss with your doctor:
- If the patient has heart disease, such as: angina pectoris (chest pain), recent myocardial infarction (heart attack), heart failure, coronary artery disease, heart valve defects, or any other heart disease, or a disease called hypertrophic cardiomyopathy with outflow obstruction (characterized by abnormal heart muscle structure).
- If the patient has narrowing of the arteries (also known as atherosclerosis); if the patient has high blood pressure or an aneurysm (abnormal dilation of a blood vessel wall).
- If the patient has arrhythmias, such as rapid or irregular heartbeat, rapid pulse, or palpitations, or if there is any information about abnormal heart rhythm.
- If the patient has hyperthyroidism.
- If the patient has low potassium levels in the blood.
- If the patient has liver or kidney disease.
- If the patient has diabetes (inhalation of high doses of formoterol may increase blood glucose levels, so before starting treatment with this medicine, and from time to time during treatment, additional blood tests may be necessary to measure blood sugar levels).
- If the patient has a pheochromocytoma (a tumor of the adrenal gland).
- If the patient is scheduled for general anesthesia. Depending on the type of anesthesia, it may be necessary to discontinue Aerox at least 12 hours before anesthesia.
- If the patient is being treated or has been treated for tuberculosis or if the patient has a viral or fungal infection in the chest.
- If the patient needs to avoid consuming alcohol for any reason.
Before using Aerox, always inform your doctor if any of the above warnings apply to you.
Before using an inhaled medicine, consult your doctor or pharmacist if you have any current or past health problems or allergies, or if you are in doubt whether Aerox can be used.
Treatment with beta-2 agonists, such as formoterol contained in Aerox, may cause a sudden decrease in blood potassium levels (hypokalemia).
Particular caution is required in patients with severe asthma,as oxygen deficiency in the blood, as well as the use of other medicines with Aerox, such as heart disease or high blood pressure medicines called diuretics, or other asthma medicines, may increase the decrease in blood potassium levels. For this reason, your doctor may occasionally recommend checking your blood potassium levels.
If you are taking high doses of inhaled corticosteroids for a long time, you may be more likely to need corticosteroids during stressful situations. Stressful situations may include: hospitalization after an accident, severe injury, or scheduled surgery. In such situations, your doctor will decide whether to increase the dose of corticosteroids or recommend other steroids in the form of tablets or injections.
If you need to be hospitalized,you should take all your medicines and inhalers, including Aerox, and any over-the-counter medicines, if possible, in their original packaging.
If you experience blurred vision or other vision disturbances, contact your doctor. Children and adolescents
Aerox should not be used in children and adolescents under 18 years of age, until more data are available.
Aerox and other medicines:
- Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription. This is necessary because Aerox may affect the action of some other medicines. Similarly, some medicines may affect the action of Aerox.
In particular, tell your doctor, pharmacist, or nurse if you are taking:
- Some medicines may enhance the effect of Aerox, and your doctor may want to closely monitor your condition while taking such medicines (including some medicines used to treat HIV infection: ritonavir, cobicistat).
- Beta-adrenergic receptor blockers. Beta-adrenergic receptor blockers are medicines used to treat many diseases, including heart disease, high blood pressure, and glaucoma (increased pressure in the eye). If beta-adrenergic receptor blockers need to be administered (including eye drops), the effect of formoterol may be reduced or formoterol may not work at all.
- Medicines that stimulate beta-adrenergic receptors (medicines that work in the same way as formoterol) may enhance the effect of formoterol.
- Medicines used to treat irregular heart rhythms (quinidine, disopyramide, procainamide).
- Medicines used to treat allergies (antihistamines).
- Medicines used to treat symptoms of depression or mental disorders, such as monoamine oxidase inhibitors (e.g., phenelzine and isocarboxazid), tricyclic antidepressants (e.g., amitriptyline and imipramine), phenothiazines.
- Medicines used to treat Parkinson's disease (L-dopa).
- Medicines used to treat hypothyroidism (L-thyroxine).
- Medicines containing oxytocin (which induce uterine contractions).
- Medicines used to treat mental disorders, such as monoamine oxidase inhibitors (MAOIs) or medicines with similar properties, such as furazolidone and procarbazine.
- Medicines used to treat heart disease (digoxin).
- Other medicines used to treat asthma (theophylline, aminophylline, or steroids).
- Diuretics.
You should also inform your doctor if you are scheduled to undergo general anesthesia for surgery or a dental procedure.
Pregnancy, breastfeeding, and fertility
There are no clinical data on the use of Aerox during pregnancy.
Aerox should not be used if you are pregnant, think you may be pregnant, or plan to have a baby, or if you are breastfeeding, unless your doctor decides otherwise.
Driving and using machines
It is unlikely that Aerox will affect your ability to drive or use machines.
Aerox contains alcohol
Aerox contains 7 mg of alcohol (ethanol) per actuation, which is equivalent to 0.20 mg/kg body weight per dose, when two actuations are used. The amount of alcohol in two actuations of this medicine is equivalent to less than 1 ml of beer or wine. The small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Aerox
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Asthma
Your doctor will regularly check that you are taking the optimal dose of Aerox.
Your doctor will determine the smallest dose that provides the best control of asthma symptoms.
Your doctor may prescribe Aerox in two different ways: a) Aerox should be used daily to treat asthma, along with another inhaled medicine that provides quick relief in case of sudden asthma symptoms, such as an attack of
shortness of breath, wheezing, and cough
Adults and the elderly:
The recommended dose is one or two actuations twice daily. The maximum daily dose is 4 actuations.
Remember: Always carry a quick-acting inhaled medicine with you to treat sudden asthma symptoms or an asthma attack.
b) Aerox should be used daily to treat asthma, as well as to provide quick relief in case of sudden asthma symptoms, such as an attack of
shortness of breath, wheezing, and cough
Adults and the elderly:
The recommended dose is one actuation in the morning and one actuation in the evening.
You should also use Aerox as a quick-acting inhaled medicine in case of sudden asthma symptoms.
If you experience asthma symptoms, use one actuation and wait a few minutes.
If there is no improvement, use another actuation.
Do not use more than 6 quick-relief actuations in 24 hours.
The maximum daily dose of Aerox, when used as the only inhaled medicine for asthma, is 8 actuations.
If you need to take more actuations to control your asthma symptoms, you should consult your doctor. A change in treatment may be necessary.
Use in children and adolescents under 18 years of age:
This medicine should NOT be used in children and adolescents under 18 years of age.
Chronic obstructive pulmonary disease (COPD)
Adults and the elderly:
The recommended dose is two actuations in the morning and two actuations in the evening.
Special patient groups:
No dose adjustment is necessary in elderly patients. There is no information on the use of Aerox in patients with liver or kidney impairment.
The dose of beclometasone dipropionate in Aerox, which is effective in treating asthma, may be lower than the dose contained in other inhaled medicines containing beclometasone dipropionate. If you have previously used another inhaled medicine containing
beclometasone dipropionate, your doctor will recommend the appropriate dose of Aerox.
Do not increase the dose.
If you feel that the effect of the medicine is too weak, always consult your doctor before increasing the dose.
In case of increased breathing difficulties:
If you experience increased breathing difficulties or wheezing immediately after inhaling the medicine, stop using Aerox immediately and use a quick-acting inhaled medicineto relieve symptoms. Consult your doctor as soon as possible.
See also section 4, "Possible side effects".
In case of worsening asthma symptoms:
If your symptoms worsen or you have difficulty controlling them (e.g., if you need to use another inhaled medicine or Aerox as a quick-relief medicine more frequently), or if the quick-relief medicine or Aerox does not relieve your symptoms, you should consult your doctor immediately. This may indicate that your asthma is worsening, and your doctor may decide to change the dose of Aerox or prescribe another treatment.
Method of administration:
Aerox is intended for inhalation use.
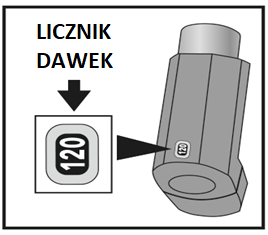
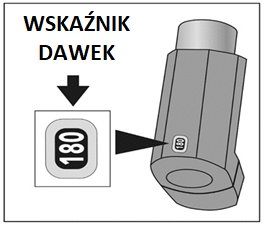
This medicine is in a pressurized container, in a plastic housing with a mouthpiece. At the back of the inhaler for 120 doses, there is a dose counter, and for 180 doses, there is a dose indicator, which shows how many doses of medicine are left.
For the 120-dose size, each time the inhaler is pressed, a dose of medicine is released, and the dose counter will show one dose less. Be careful not to drop the inhaler, as this may cause the dose counter to decrement.
For the 180-dose size, the dose indicator will show the approximate number of actuations left in the container. The dose indicator window displays the number of actuations left in the inhaler in increments of twenty (e.g., 180, 120, 100, 80, etc.). When 20 doses are left, and the dose indicator shows 20, it means that the container is near the end of its life.
After 180 actuations, the dose indicator will show 0.
The dose indicator will stop moving at "0".
Testing the inhaler
Before first use, or if the inhaler has not been used for 14 days or more, you should perform an inhaler test to ensure it is working properly.
- Remove the protective cap from the mouthpiece.
- Hold the container in an upright position with the mouthpiece pointing downwards.
- Point the mouthpiece "away from you" and press the container firmly to release one actuation.
- If the inhaler has not been used for 14 days or more, press the container firmly once to release an actuation.
- For the 120-dose size, check the dose counter. If the inhaler is being tested for the first time, the dose counter should show 120.
- For the 180-dose size, check the dose indicator. If the inhaler is being tested for the first time, the dose indicator should show 180.
How to use the inhaler
If possible, stand or sit upright while inhaling.
Before inhaling, check the dose counter or dose indicator, which shows how many doses are left. If the dose counter or dose indicator shows "0", there are no doses left – discard the inhaler and purchase a new one.


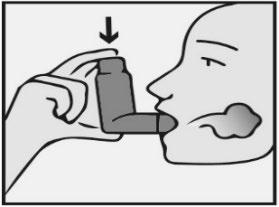
- 1) 2) 3) 4) 5)
- 1. Remove the protective cap from the mouthpiece and check that the mouthpiece is clean and free of dust or other contaminants (Figure 1).
- 2. Exhale slowly and deeply (Figure 2).
- 3. Hold the container in an upright position, with the base pointing upwards, and then place the mouthpiece between your lips. Do not bite the mouthpiece (Figure 3).
- 4. Take a deep and slow breath in through your mouth. As soon as you start breathing in, press the top of the inhaler firmlyto release one actuation of the medicine. If you have a weak grip, it may be easier to hold the inhaler with both hands: place both index fingers on the top of the inhaler and both thumbs on the bottom (Figure 4).
- 5. Hold your breath for as long as possible, then remove the inhaler from your mouth and breathe out slowly. Do not breathe out into the inhaler (Figure 5).
If you need to take another dose, hold the inhaler upright for about half a minute, then repeat the steps from 2 to 5.
Important:Do not perform the actions in steps 2 to 5 too quickly.
After use, replace the protective cap and check the dose counter for the 120-dose size and the dose indicator for the 180-dose size.
To reduce the risk of fungal infection of the mouth and throat, rinse your mouth or throat with water after each inhalation, or brush your teeth.
When to replace the inhaler
If the dose counter or dose indicator shows 20, you should have a new inhaler ready. Stop using the inhaler when the dose counter or dose indicator shows 0, as the medicine left in the container may not provide a full dose.
If a "mist" appears over the top opening of the mouthpiece or from the side of the mouth, it means that Aerox is not reaching the lungs as it should. Take another dose, following the instructions, starting again from step 2.
If you feel that the effect of Aerox is too strong or too weak, consult your doctor or pharmacist.
If you have difficulty using the inhaler during inhalation, you can use a spacer (AeroChamber Plus). Ask your doctor, pharmacist, or nurse about this device.
It is essential to read the leaflet accompanying the AeroChamber Plus spacer and follow the instructions for use and cleaning recommendations.
Cleaning
Clean the inhaler once a week.
Do not remove the container from the plastic housing, and do not use water or other liquids to clean the inhaler.
Cleaning the inhaler:
- 1. Remove the protective cap from the mouthpiece by pulling it off the inhaler.
- 2. Wipe the mouthpiece and dose indicator from the inside and outside with a dry cloth or tissue.
- 3. Replace the protective cap.
Using a higher dose of Aerox than recommended:
- Using a higher dose of formoterol than recommended may cause the following symptoms: nausea, vomiting, rapid heartbeat, palpitations, arrhythmias, changes in electrocardiogram (ECG), headache, tremors, drowsiness, excessive acidic products in the blood, decreased potassium levels in the blood, increased glucose levels in the blood. Your doctor may recommend a blood test to check your potassium and glucose levels.
- Using too high a dose of beclometasone dipropionate may cause short-term adrenal gland disorders, which resolve on their own within a few days. Your doctor may recommend a blood test to check your cortisol levels.
If you experience any of these symptoms, inform your doctor.
Missing a dose of Aerox:
Take the missed dose as soon as possible. If it is almost time for your next dose, do not take the missed dose, just take the next dose at the usual time. Do not take a double dose to make up for a missed dose.
Stopping treatment with Aerox:
Even if you feel better, do not stop using Aerox or reduce the dose without consulting your doctor. It is essential to take the medicine regularly, even when your symptoms have improved.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Aerox can cause side effects, although not everybody gets them.
As with other inhaled medicines, there is a risk of worsening shortness of breath and wheezing immediately after using Aerox, known as paradoxical bronchospasm. You should stop using Aerox immediatelyand use a quick-acting inhaled bronchodilatorto relieve symptoms. Consult your doctor as soon as possible.
Inform your doctor immediatelyif you experience any allergic reactions, such as:
skin allergies, itching, skin rash, skin redness, swelling of the skin or mucous membranes, especially of the eyes, face, lips, and throat.
Other possible side effects are listed below by frequency of occurrence.
- Fungal infections (of the mouth and throat),
- headache,
- hoarseness,
- throat pain.
Pneumonia in patients with COPD: Tell your doctor if you experience any of the following symptoms while using Aerox; these may be symptoms of a lung infection:
- fever or chills,
- increased production of sputum or change in sputum color,
- worsening cough or increased breathing difficulties.
Uncommon(occurring in less than 1 in 100 patients):
- rapid heartbeat, irregular heartbeat, and arrhythmias,
- changes in electrocardiogram (ECG),
- flu-like symptoms,
- sinusitis,
- nasal congestion,
- ear infection,
- throat irritation,
- cough and cough with sputum production,
- asthma attack,
- vaginal fungal infections,
- nausea,
- taste disturbances,
- mouth burning,
- dry mouth,
- difficulty swallowing,
- indigestion,
- stomach upset,
- diarrhea,
- muscle pain and muscle cramps,
- redness of the face and throat,
- increased blood flow to some tissues,
- increased sweating,
- tremors,
- restlessness, especially movement-related,
- dizziness,
- hives,
- changes in some blood test results: o decreased white blood cell count, o increased platelet count, o decreased potassium levels in the blood, o increased glucose levels in the blood, o increased insulin, free fatty acids, and ketone bodies in the blood.
The following side effects have also been reported as "uncommon" in patients with chronic obstructive pulmonary disease:
- decreased cortisol levels in the blood, which is caused by the effect of corticosteroids on the adrenal glands,
- irregular heartbeat.
Rare(occurring in less than 1 in 1000 patients):
- feeling of pressure in the chest,
- arrhythmias (caused by premature contraction of the heart),
- decreased blood pressure,
- increased blood pressure,
- kidney inflammation,
- swelling of the skin and mucous membranes, lasting for several days.
Very rare(occurring in less than 1 in 10,000 patients):
- shortness of breath,
- asthma worsening,
- decreased platelet count,
- swelling of the hands and feet.
Long-term use of inhaled corticosteroids in high doses may rarely cause
systemic effects, including:
- adrenal gland disorders (adrenal gland suppression),
- decreased bone density (osteoporosis),
- growth retardation in children and adolescents,
- increased eye pressure (glaucoma),
- cataracts.
Unknown frequency(frequency cannot be estimated from available data):
- sleep disturbances,
- depression or feeling down,
- nervousness,
- excessive excitement or irritability.
These side effects are more likely to occur in children, but the frequency of these side effects is unknown.
- Blurred vision,
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. You can also report side effects directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: + 48 22 49 21 301
fax: + 48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
You can also report side effects to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Aerox
Keep the medicine out of the sight and reach of children.
Single packaging containing one container with 120 or 180 doses
For the pharmacist:
Store the inhaler in the refrigerator (2°C – 8°C) for a maximum of 18 months.
Write the date of issue of the medicine to the patient on the self-adhesive label on the packaging and attach the label to the inhaler. Ensure that the period between the date of issue of the medicine and the expiry date stated on the packaging is at least 3 months.
For the patient:
Do not store the inhaler at temperatures above 25°C.
Do not use Aerox after 3 months from first use and after the expiry date stated on the carton or label. The expiry date refers to the last day of the given month.
Double or triple packaging containing two or three containers with 120 doses
Before first use:store the inhalers in the refrigerator (2°C – 8°C).
After first use:do not store the inhalers at temperatures above 25°C. Store for a maximum of 3 months.
When starting to use each inhaler, write the date of first use on the self-adhesive label on the packaging and attach the label to the inhaler. Do not use this medicine after 3 months from first use and after the expiry date stated on the carton or label.
The expiry date refers to the last day of the given month.
Do not freeze.
If the inhaler has been cooled, warm it in your hands for a few minutes before use. Never use other methods to heat the container.
Important:The container contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not pierce the container.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Aerox contains:
The active substances of the medicine are: beclometasone dipropionate and formoterol fumarate dihydrate.
One actuation from the inhaler contains 100 micrograms of beclometasone dipropionate and 6 micrograms of formoterol fumarate dihydrate. This corresponds to a delivered dose from the mouthpiece containing 84.6 micrograms of beclometasone dipropionate and 5.0 micrograms of formoterol fumarate dihydrate.
The other ingredients are: anhydrous ethanol, concentrated hydrochloric acid, norflurane (HFA 134a).
This medicine contains fluorinated greenhouse gases.
Each 120-dose inhaler contains 8.15 g of HFA 134a, which corresponds to 0.012 tons of CO2 equivalent (global warming potential GWP = 1,430).
Each 180-dose inhaler contains 11.2 g of HFA 134a, which corresponds to 0.016 tons of CO2 equivalent (global warming potential GWP = 1,430).
What Aerox looks like and contents of the pack:
Aerox is available as a solution for inhalation, in an aluminum container with a metering valve, mounted in a white plastic actuator, which contains a dose counter (120 doses) or a dose indicator (180 doses) with a pink plastic protective cap.
Each pack contains:
1 pressurized container providing 120 doses (actuations) or
2 pressurized containers, each providing 120 doses or
3 pressurized containers, each providing 120 doses or
1 pressurized container providing 180 doses
Not all pack sizes may be marketed.
Marketing authorization holder
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Tel: +48 22 732 77 00
Manufacturer
Genetic S.p.A.
Contrada Canfora
84084 Fisciano
Italy
This medicine is authorized in the Member States of the European Economic Area under the following names:
| Austria | Beclometason/Formoterol Genetic 100 mikrogramm /6 mikrogramm/Sprühstoß Druckgasinhalation, Lösung |
| Belgium | Beclometasone/Formoterol Genetic 100/6 microgram/dosis aërosol, oplossing |
| Luxembourg | Beclometasone/Formoterol Genetic 100 microgrammes /6 microgrammes/dose, solution pour inhalation en flacon pressurisé |
Date of last revision of the leaflet:
| Germany | Beclometason/Formoterol Genetic 100 Mikrogramm/6 Mikrogramm pro Inhalation Druckgasinhalation, Lösung |
| Netherlands | Beclometason/Formoterol Allgen 100 microgram /6 microgram/dosis, aërosol, oplossing |
| Estonia | Beclametasone/Formoterol Genetic |
| France | BÉCLOMÉTASONE/FORMOTÉROL BIOGARAN 100 microgrammes /6 microgrammes/dose, solution pour inhalation en flacon pressurisé |
| Lithuania | Beclometasone/Formoterol Genetic 100 mikrogramų /6 mikrogramai spūsnyje suslėgtasis įkvepiamasis tirpalas |
| Latvia | Beclometasone/Formoterol Genetic 100 mikrogrami/6 mikrogrami izsmidzinājumā aerosols inhalācijām, zem spiediena, šķīdums |
| Romania | Beclometazonă/Formoterol Genetic 100 micrograme /6 micrograme pe doză, soluţie de inhalat presurizată |
| Slovakia | Beklometasón-formoterol Genetic 100 mikrogramov /6 mikrogramov/dávka |
| Italy | Beclometasone e Formoterolo Genetic |
| Portugal | Beclometasona Formoterol Genetic 100 mcg/6 mcg Solução pressurizada para inalação |
| Spain | Beclometasona Formoterol Genetic 100 microgramos/6 microgramos/pulsación Solución para inhalación en envase a presión |
| Greece | Breair |
| Poland | Aerox |
| Hungary | Beclometasone formoterol Genetic 100 mcg /6 mcg túlnyomásos inhalációs oldat |
| Bulgaria | Беклометазон формотерол Genetic 100 микрограма /6 микрограма/доза, разтвор под налягане за инхалация |
| Czech Republic | Beklometason/Formoterol Genetic |
| Slovenia | Beklometazon/formoterol Genetic 100 mikrogramov /6 mikrogramov na potisk inhalacijska raztopina pod tlakom |
| Ireland | Beclometasone/formoterol 100 microgram/60 microgram per actuation pressurised inhalation solution |
| Croatia | Beklometazondipropionat/formoterolfumarat dihidrat Genetic 100 mikrograma /6 mikrograma po potisku, stlačeni inhalat, otopina |
| Malta | Beclometasone formoterol Genetic 100 microgram/6 microgram per actuation pressurized inhalation solution |
| Cyprus | Beclometasone formoterol Genetic 100 microgram/6 microgram per actuation pressurized inhalation solution |
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGenetic S.p.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AeroxDosage form: Aerosol, (200 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription requiredDosage form: Aerosol, (100 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription not requiredDosage form: Aerosol, (200 mcg + 6 mcg)/doseActive substance: formoterol and beclometasonePrescription not required
Alternatives to Aerox in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aerox in Spain
Alternative to Aerox in Ukraine
Online doctors for Aerox
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aerox – subject to medical assessment and local rules.














