Flutixon

Ask a doctor about a prescription for Flutixon

How to use Flutixon
Leaflet accompanying the packaging: patient information
Flutixon, 125 micrograms/inhalation dose, powder for inhalation in a hard capsule
Flutixon, 250 micrograms/inhalation dose, powder for inhalation in a hard capsule
Fluticasone propionate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Flutixon and what is it used for
- 2. Important information before using Flutixon
- 3. How to use Flutixon
- 4. Possible side effects
- 5. How to store Flutixon
- 6. Contents of the packaging and other information
1. What is Flutixon and what is it used for
Flutixon belongs to a group of medicines used in obstructive airway diseases (diseases with reduced airflow in the lungs). The active substance of the medicine, fluticasone propionate, is a corticosteroid (adrenal cortex hormone) with local anti-inflammatory effects in the lungs.
Flutixon is indicated:
- for the prevention of asthma attacks in adults and children over 16 years of age:
- mild asthma - in patients who require daily symptomatic treatment with bronchodilators;
- moderate asthma - unstable or worsening asthma, despite regular use of asthma preventive medications or only bronchodilators;
- severe asthma - in patients with severe chronic asthma and those who require oral steroids to control asthma symptoms. Starting fluticasone propionate in many people allows for a reduction in the doses of oral steroids or their complete withdrawal;
- for the treatment of symptoms of chronic obstructive pulmonary disease (COPD).
Flutixon should not be used to interrupt a sudden asthma attack or wheezing in the airways. For this purpose, a quick-acting bronchodilator (e.g., salbutamol) should be used, which the patient should always carry with them. Be careful not to confuse Flutixon with a bronchodilator used as needed.
2. Important information before using Flutixon
When not to use Flutixon
- if the patient has been diagnosed with hypersensitivity (allergy) to fluticasone propionate or any other component of Flutixon.
Warnings and precautions
Before starting Flutixon, consult a doctor or pharmacist.
When to be particularly careful when using Flutixon
- in the first months after switching from oral corticosteroids to inhaled Flutixon, as the change in treatment may reveal symptoms of allergy, such as allergic rhinitis or rash, which were previously treated with oral corticosteroids; in such cases, consult a doctor;
- in case of sudden discontinuation of treatment or reduction of the dose of the medicine, as well as during periods of increased stress, infection, or severe injury (e.g., due to an accident), recent surgery, or planned surgery, additional side effects may occur, such as stomach pain, fatigue, loss of appetite, nausea, diarrhea, weight loss, headache, or drowsiness, low potassium levels in the blood, low blood pressure, or seizures; to prevent these symptoms, the doctor may prescribe additional corticosteroids at that time;
- when taking high doses of Flutixon for a long time, suppression of natural steroid hormone production by the adrenal glands may occur, which can lead to decreased bone mass, cataracts, weight gain, rounded face (moon face), high blood pressure, slowed growth in children and adolescents; if such symptoms are observed, consult a doctor;
- if symptoms such as fatigue, loss of appetite, nausea, vomiting, weight loss, abdominal pain, headache, decreased blood sugar levels, seizures, loss of consciousness, or low blood pressure occur, as they may be signs of adrenal insufficiency or acute adrenal crisis; in such cases, seek medical attention immediately;
- in patients with active or past tuberculosis;
- in patients with a history of diabetes; in diabetic patients, it may be necessary to monitor blood sugar levels more frequently and adjust the dose of antidiabetic medications being taken.
- if the patient experiences blurred vision or other vision disturbances, they should consult a doctor.
During Flutixon treatment, asthma exacerbations or side effects related to asthma may occur. If asthma symptoms worsen after starting Flutixon or are not properly treated, the patient should continue treatment and consult a doctor.
Children
Flutixon is not intended for use in children under 16 years of age.
Flutixon and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, including those available without a prescription, as well as any you plan to take. Only use medicines with Flutixon that have been prescribed by a doctor.
Tell your doctor if you have recently taken oral corticosteroids.
Some medicines used with Flutixon may increase the risk of side effects, such as:
- certain antiviral medicines (e.g., ritonavir, used to treat HIV infections);
- certain antifungal medicines (e.g., ketoconazole, itraconazole).
Some medicines may enhance the effects of Flutixon, and the doctor may want to closely monitor the patient's condition when taking such medicines (including certain HIV medicines: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult a doctor before using this medicine.
Pregnancy
Do not use Flutixon during pregnancy without consulting a doctor.
Breastfeeding
Do not use Flutixon during breastfeeding without consulting a doctor.
Driving and using machines
Flutixon does not affect the ability to drive or use machines.
Flutixon contains lactose
Each dose of Flutixon contains approximately 25 mg of lactose. In people with lactose intolerance, this amount of lactose usually does not cause any problems.
3. How to use Flutixon
This medicine should always be used as directed by a doctor. If you have any doubts, consult a doctor or pharmacist.
Flutixon is intended for inhalation use only with an inhaler. Do not swallow the capsules.
Detailed instructions for using the medicine can be found at the end of the leaflet.
It is essential to use Flutixon daily, unless the doctor advises otherwise.
The therapeutic effect occurs within 4 to 7 days.
In case of worsening asthma symptoms or decreased treatment effectiveness- asthma or COPD - (if there is worsening wheezing, or if it is necessary to use a quick-acting bronchodilator more frequently) do not increase the number of Flutixon inhalations. Consult a doctor immediately, who will prescribe the appropriate treatment.
Warning: Follow the recommended dosage of Flutixon.
Asthma:
Adults and children over 16 years of age:
The dose of the medicine depends on the severity of the disease and the individual patient's response to treatment:
Mild asthma: 125 micrograms twice a day, i.e., 1 inhalation of Flutixon 125 micrograms twice a day.
Moderate asthma: 125 micrograms to 250 micrograms twice a day, i.e., 1 to 2 inhalations of Flutixon 125 micrograms twice a day or 1 inhalation of Flutixon 250 micrograms twice a day.
Severe asthma: 250 micrograms to 500 micrograms twice a day, i.e., 2 to 4 inhalations of Flutixon 125 micrograms twice a day or 1 to 2 inhalations of Flutixon 250 micrograms twice a day.
In case of controlled asthma symptoms, the doctor may reduce the dosage, depending on the response to treatment, to achieve optimal treatment effectiveness with the lowest daily dose of the medicine.
Flutixon is not intended for use in children under 16 years of age.
Chronic obstructive pulmonary disease (COPD): Adults
250 micrograms twice a day, i.e., 2 inhalations of Flutixon 125 micrograms twice a day or 1 inhalation of Flutixon 250 micrograms twice a day.
Improvement occurs after about 6 months of treatment. If there is no improvement, the doctor should re-evaluate the patient clinically.
To achieve optimal effects of Flutixon, it should be used daily.
Special patient groups:
There is no need to adjust the dosage in elderly patients or those with renal or hepatic impairment.
Using a higher dose of Flutixon than recommended
In case of using a higher dose of Flutixon than recommended, consult a doctor or pharmacist immediately.
Long-term use of higher doses of Flutixon than recommended may lead to a decrease in the production of steroid hormones by the adrenal glands.
Missing a dose of Flutixon
In case of missing a dose of Flutixon, take it as soon as possible, and then continue taking the medicine as directed by the doctor.
Do not take a double dose to make up for the missed dose.
Stopping Flutixon treatment
Flutixon should be taken until the doctor advises to stop.
Do not stop Flutixon treatment suddenly, as the disease symptoms may worsen.
In case of doubts about the use of the medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Flutixon can cause side effects, although not everybody gets them.
During Flutixon treatment, the following side effects may occur:
Very common (occurring in more than 1 in 10 people)
- white patches in the mouth and throat (thrush), rinsing the mouth with water (without swallowing) after each inhalation may be helpful. The doctor may also prescribe a medicine to treat thrush.
Common (occurring in more than 1 in 100 and less than 1 in 10 people):
- hoarseness, loss of voice, rinsing the mouth with water (without swallowing) after each inhalation may be helpful.
- pneumonia (lung infection) in patients with COPD (frequent side effect). Tell your doctor if any of the following symptoms occur during fluticasone propionate treatment; they may be signs of a lung infection:
- fever or chills
- increased mucus production
- worsening cough or increased breathing difficulties
- easy bruising.
Uncommon (occurring in more than 1 in 1000 and less than 1 in 100 people)
- skin hypersensitivity reactions (e.g., rash).
Rare (occurring in less than 1 in 10,000 people)
- swelling of the face, lips, tongue, or throat, which can cause difficulty breathing or swallowing (angioedema),
- severe allergic reactions (anaphylactic reactions),
- difficulty breathing, wheezing, coughing, gasping (paradoxical bronchospasm),
If you notice such symptoms or if they occur suddenly after inhalation, use a quick-acting inhaled bronchodilator, stop using Flutixon, and consult a doctor, as this may indicate
hypersensitivity to this medicine.
- Flutixon may interfere with the production of steroid hormones in the body, especially when taking high doses of the medicine for a long time. Symptoms include: slowed growth in children and adolescents; decreased bone mass; eye diseases (cataracts, glaucoma); weight gain, high blood pressure, rounded face (Cushing's syndrome).
- increased blood sugar levels (hyperglycemia),
- joint pain,
- nausea,
- restlessness, sleep disturbances, and changes in behavior, including increased activity and irritability (mainly in children).
Frequency not known (frequency cannot be estimated from available data)
- blurred vision.
If any side effect worsens or if any side effects not listed in this leaflet occur, tell your doctor or pharmacist.
Reporting side effects
If side effects occur, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw,
phone: +48 22 49 21 301,
fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Flutixon
Store below 30°C.
Store out of sight and reach of children.
Do not use Flutixon after the expiry date stated on the carton. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Flutixon contains
The active substance of the medicine is fluticasone propionate. One hard capsule of the medicine contains 125 or 250 micrograms (µg) of fluticasone propionate.
Other ingredients:
Flutixon 125 µg: lactose anhydrous, lactose monohydrate, HPMC #3 (hypromellose) capsule.
Flutixon 250 µg: lactose anhydrous, lactose monohydrate, HPMC #3 (hypromellose) capsule, black ink.
What Flutixon looks like and contents of the pack
Hard capsule 125 µg: colorless, containing a white or almost white powder.
Hard capsule 250 µg: colorless, with a black ring, containing a white or almost white powder.
Available packaging:
60 capsules or 120 capsules in blisters and a cardboard box, with an inhaler.
Marketing authorization holder and manufacturer
Marketing authorization holder
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Manufacturer
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Date of last revision of the leaflet: 04.2023
Instructions for using the inhaler
The doctor or pharmacist should instruct the patient on how to use the Flutixon powder for inhalation in hard capsules.
How to use the inhaler:
- 1. Remove the cap.
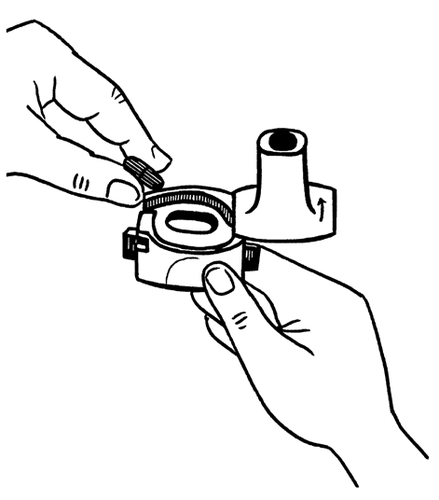
- 2. Hold the base of the inhaler firmly and open it by twisting the mouthpiece in the direction indicated by the arrow.
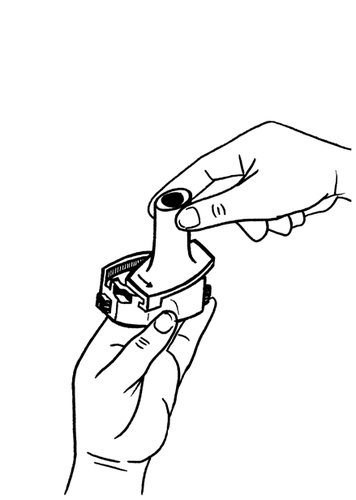
- 3.Place the capsule in the capsule-shaped compartment, which is located in the base of the inhaler.
The capsule should be removed from the blister pack immediately before use.
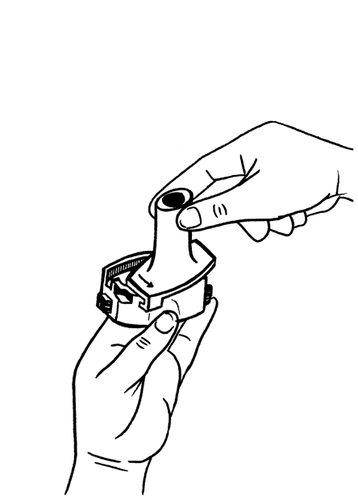
- 4. Twist the mouthpiece back to the closed position.
- 5. Press the buttons to the stop ( only once), holding the inhaler in a vertical position. NOTE: at this moment, the capsule may break and small pieces may enter the mouth or throat. Swallowing them is not harmful. The likelihood of this happening is minimal if the capsule is not pierced more than once, storage conditions are maintained, and the capsule is removed from the blister pack immediately before use (see point 3).
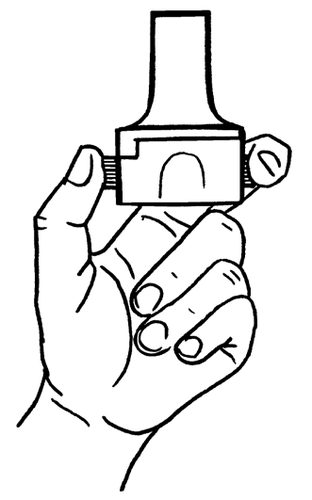
- 6. Take a deep breath out.
- 7. Place the mouthpiece in your mouth and slightly tilt your head back. Close your lips around the mouthpiece and take one quick and steady breath in. As the capsule rotates in the inhaler chamber and the powder is dispersed, a characteristic sound (rattling) should be heard. If this sound does not occur, it may mean that the capsule is stuck in the compartment. In this case, open the inhaler and, by lifting the capsule, remove it from the compartment. DO NOTlift the capsule by pressing the buttons multiple times.
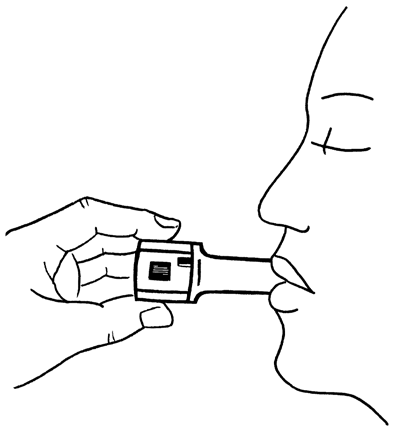
- 8. After hearing the characteristic sound (rattling), hold your breath for as long as possible without discomfort, and then remove the inhaler from your mouth. Breathe out. Open the inhaler and check if any powder is still left in the capsule. If powder is left in the capsule, repeat the steps described in points 6 to 8.
- 9. After use, open the inhaler, remove the empty capsule, close the mouthpiece, and put the cap back on.
Cleaning the inhaler:
To remove powder residue, wipe the mouthpiece and capsule compartment with a dry cloth or a clean, soft brush.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAdamed Pharma S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FlutixonDosage form: Powder, 250 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Powder, 500 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Powder, 50 mcg/dose inh.Active substance: fluticasonePrescription required
Alternatives to Flutixon in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Flutixon in Україна
Alternative to Flutixon in Іспанія
Online doctors for Flutixon
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Flutixon – subject to medical assessment and local rules.