Flutixon

Ask a doctor about a prescription for Flutixon

How to use Flutixon
Leaflet attached to the packaging: patient information
Flutixon, 125 micrograms/inhalation dose, powder for inhalation in a hard capsule
Flutixon, 250 micrograms/inhalation dose, powder for inhalation in a hard capsule
Fluticasone propionate
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Flutixon and what is it used for
- 2. Important information before using Flutixon
- 3. How to use Flutixon
- 4. Possible side effects
- 5. How to store Flutixon
- 6. Contents of the packaging and other information
1. What is Flutixon and what is it used for
Flutixon belongs to a group of medicines used in obstructive airway diseases (diseases with reduced airflow in the lungs). The active substance of the medicine, fluticasone propionate, is a corticosteroid (adrenal cortex hormone) with local anti-inflammatory action in the lungs.
Flutixon is indicated:
- for the prevention of asthma attacks in adults and children over 16 years of age:
- mild asthma - in patients who require daily symptomatic treatment with bronchodilators;
- moderate asthma - unstable or worsening asthma, despite regular use of asthma preventive medications or only bronchodilators;
- severe asthma - in patients with severe chronic asthma and requiring oral steroids to control asthma symptoms. Starting fluticasone propionate in many people allows for a reduction in the doses of oral steroids or their complete withdrawal;
- for the treatment of symptoms of chronic obstructive pulmonary disease (COPD).
Flutixon should not be used to interrupt a sudden asthma attack or wheezing in the airways. For this purpose, a quick-acting bronchodilator (e.g., salbutamol) should be used, which the patient should always carry with them. Be careful not to confuse Flutixon with a bronchodilator used as needed.
2. Important information before using Flutixon
When not to use Flutixon
- if the patient has been diagnosed with hypersensitivity (allergy) to fluticasone propionate or any of the other ingredients of Flutixon.
Warnings and precautions
Before starting to take Flutixon, you should discuss it with your doctor or pharmacist.
When to be particularly careful when using Flutixon
- in the first months after changing treatment from oral corticosteroids to inhaled Flutixon, as the change in treatment may reveal symptoms of allergy, such as allergic rhinitis or rash, which were previously treated with oral corticosteroids; in such a case, you should consult a doctor;
- in case of sudden discontinuation of treatment or reduction of the dose of the medicine, as well as during periods of increased stress, infection, or severe injury (e.g., caused by an accident), recent surgery, or planned surgery, additional side effects may occur, such as stomach pain, fatigue, loss of appetite, nausea, diarrhea, weight loss, headache, or drowsiness, low potassium levels in the blood, low blood pressure, or seizures; to prevent the occurrence of these symptoms, the doctor may prescribe additional corticosteroids at that time;
- when taking large doses of Flutixon for a long time, suppression of natural steroid hormone production by the adrenal glands may occur, which can lead to a decrease in bone mass, cataracts, weight gain, high blood pressure, slowing of growth in children and adolescents, and if such symptoms are observed, you should consult a doctor;
- if symptoms such as fatigue, loss of appetite, nausea, vomiting, weight loss, abdominal pain, headache, decreased blood sugar levels, seizures, loss of consciousness, or low blood pressure occur, as this may be a sign of adrenal insufficiency or acute adrenal crisis; you should then seek medical attention immediately;
- in patients with active or past tuberculosis;
- in patients with a history of diabetes; in diabetic patients, it may be necessary to monitor blood sugar levels more frequently and adjust the dose of antidiabetic medications being taken.
- if the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
During the use of Flutixon, asthma exacerbations or side effects related to asthma may occur. If, after starting Flutixon, the symptoms of asthma worsen or are not properly treated, the patient should continue treatment and contact their doctor.
Children
Flutixon is not intended for use in children under 16 years of age.
Flutixon and other medicines
You should tell your doctor about all medicines you are currently taking or have recently taken, including those that are available without a prescription, as well as any that you plan to take. You should only use Flutixon with medicines that your doctor has prescribed.
You should inform your doctor if you have recently taken oral corticosteroids (by mouth or by injection).
Some medicines used at the same time as Flutixon may increase the risk of side effects, e.g.,
- certain antiviral medicines (e.g., ritonavir - used to treat HIV infections);
- certain antifungal medicines (e.g., ketoconazole, itraconazole).
Some medicines may enhance the effect of Flutixon, and your doctor may want to closely monitor your condition while taking such medicines (including certain HIV medicines: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor before using this medicine.
Pregnancy
Flutixon should not be used during pregnancy without consulting a doctor.
Breastfeeding
Flutixon should not be used during breastfeeding without consulting a doctor.
Driving and using machines
Flutixon does not affect the ability to drive or use machines.
Flutixon contains lactose
Each dose of Flutixon contains about 25 mg of lactose. In people with lactose intolerance, this amount of lactose does not usually cause any disturbances.
3. How to use Flutixon
This medicine should always be used as directed by your doctor. If you have any doubts, you should consult your doctor or pharmacist.
Flutixon is intended for inhalation use only with an inhaler. Do not swallow the capsules.
Detailed instructions for using the medicine can be found at the end of the leaflet.
It is essential to use Flutixon every day, unless your doctor advises otherwise.
The therapeutic effect occurs within 4 to 7 days.
In case of worsening of disease symptoms or decreased treatment efficacy- asthma or COPD - (if there is worsening wheezing, or if it is necessary to use a larger number of inhalations of a quick-acting bronchodilator as needed) do not increase the number of Flutixon inhalations. You should contact your doctor immediately, who will prescribe the appropriate treatment.
Warning: You should strictly follow the dosage of the medicine.
Asthma:
Adults and children over 16 years of age:
The dose of the medicine depends on the severity of the disease and the individual patient's response to treatment:
Mild asthma: 125 micrograms twice a day, i.e., 1 inhalation of Flutixon 125 micrograms twice a day.
Moderate asthma: 125 micrograms to 250 micrograms twice a day, i.e., 1 to 2 inhalations of Flutixon 125 micrograms twice a day or 1 inhalation of Flutixon 250 micrograms twice a day.
Severe asthma: 250 micrograms to 500 micrograms twice a day, i.e., 2 to 4 inhalations of Flutixon 125 micrograms twice a day or 1 to 2 inhalations of Flutixon 250 micrograms twice a day.
In case of control of asthma symptoms, the doctor may reduce the dosage, depending on the response to treatment, to achieve optimal treatment efficacy at the lowest daily dose of the medicine.
Flutixon is not intended for use in children under 16 years of age.
Chronic obstructive pulmonary disease (COPD): Adults
250 micrograms twice a day, i.e., 2 inhalations of Flutixon 125 micrograms twice a day or 1 inhalation of Flutixon 250 micrograms twice a day.
Improvement occurs after about 6 months of treatment. If there is no improvement, the doctor should re-evaluate the patient clinically.
To achieve optimal Flutixon efficacy, it should be used every day.
Special patient groups:
There is no need to change the dosage in elderly patients or patients with renal or hepatic impairment.
Using a higher dose of Flutixon than recommended
In case of using a higher dose of Flutixon than recommended, you should contact your doctor or pharmacist immediately.
Long-term use of higher doses of Flutixon than recommended may lead to a decrease in the production of steroid hormones by the adrenal glands.
Missing a dose of Flutixon
In case of missing a dose of Flutixon, you should take it as soon as possible, and then continue taking the medicine as directed by your doctor.
You should not take a double dose to make up for the missed dose.
Stopping the use of Flutixon
Flutixon should be taken as long as your doctor does not advise you to stop.
You should not suddenly stop taking Flutixon, as the symptoms of the disease may worsen.
If you have any doubts about using the medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Flutixon can cause side effects, although not everybody gets them.
During the use of Flutixon, the following side effects may occur:
Very common (occurring in more than 1 in 10 people)
- white patches in the mouth and throat (thrush), Rinsing the mouth with water (without swallowing) after each inhalation may be helpful. The doctor may also prescribe a medicine to treat thrush.
Common (occurring in more than 1 in 100 and less than 1 in 10 people):
- hoarseness, loss of voice, Rinsing the mouth with water (without swallowing) after each inhalation may be helpful.
- pneumonia (lung infection) in patients with COPD (common side effect). You should tell your doctor if any of the following symptoms occur during the use of fluticasone propionate; these may be symptoms of a lung infection:
- fever or chills
- increased production of mucus
- worsening cough or increased breathing difficulties
- easy bruising.
Uncommon (occurring in more than 1 in 1000 and less than 1 in 100 people)
- skin hypersensitivity reactions (e.g., rash).
Rare (occurring in less than 1 in 10,000 people)
- swelling of the face, lips, tongue, or throat, which can cause difficulty breathing or swallowing (angioedema),
- severe hypersensitivity reactions (anaphylactic reactions),
- difficulty breathing, wheezing, coughing, gasping (paradoxical bronchospasm),
If you notice such symptoms, or if they occur suddenly after inhalation, you should immediately use a quick-acting inhaled bronchodilator, stop using Flutixon, and contact your doctor, as this may indicate
hypersensitivity to this medicine.
- Flutixon may interfere with the production of steroid hormones in the body, especially when taking large doses of the medicine for a long time. Symptoms include: slowing of growth in children and adolescents; decreased bone mass; eye diseases (cataracts, glaucoma); weight gain, high blood pressure, rounding of the face (moon face) (Cushing's syndrome).
- increased blood sugar levels (hyperglycemia),
- joint pain,
- indigestion,
- restlessness, sleep disturbances, and changes in behavior, including increased activity and irritability (mainly in children).
Frequency not known (frequency cannot be estimated from the available data)
- blurred vision.
If any side effect worsens or if any side effects not listed in this leaflet occur, you should inform your doctor or pharmacist.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw,
phone: +48 22 49 21 301,
fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Flutixon
Store at a temperature below 30°C.
Store in a place out of sight and reach of children.
Do not use Flutixon after the expiry date stated on the carton. The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Flutixon contains
The active substance of the medicine is fluticasone propionate. One hard capsule of the medicine contains 125 or 250 micrograms (µg) of fluticasone propionate.
Other ingredients:
Flutixon 125 µg: lactose anhydrous, lactose monohydrate, HPMC #3 capsule (hypromellose).
Flutixon 250 µg: lactose anhydrous, lactose monohydrate, HPMC #3 capsule (hypromellose), black ink.
What Flutixon looks like and contents of the pack
Hard capsule 125 µg: colorless, containing a white or almost white powder.
Hard capsule 250 µg: colorless, with a black ring, containing a white or almost white powder.
Available packaging:
60 capsules or 120 capsules in blisters and a cardboard box, with an inhaler.
Marketing authorization holder and manufacturer
Marketing authorization holder
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Manufacturer
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Date of last revision of the leaflet: 04.2023
Instructions for using the inhaler
The doctor or pharmacist should instruct the patient on how to use the Flutixon powder for inhalation in hard capsules.
How to use the inhaler:
- 1. Remove the cap.
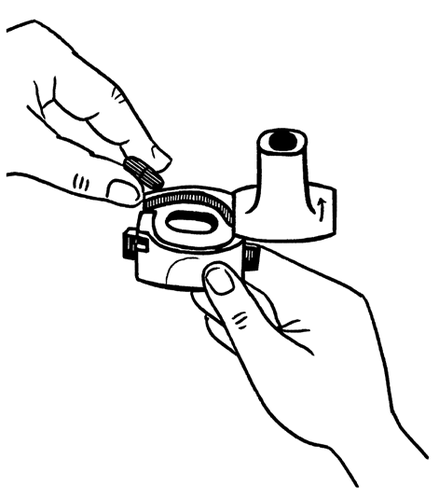
- 2. Hold the base of the inhaler firmly and open it by turning the mouthpiece in the direction indicated by the arrow.
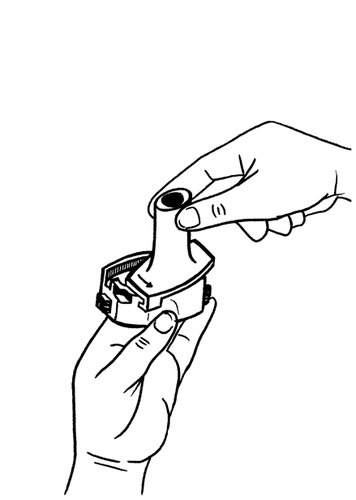
- 3.Place the capsule in the compartment shaped like a capsule, which is located in the base of the inhaler.
The capsule should be removed from the blister pack immediately before use.
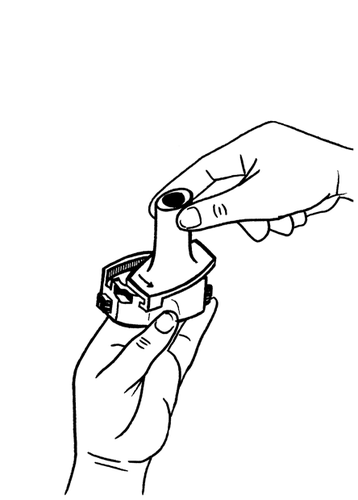
- 4. Turn the mouthpiece back to the closed position.
- 5. Press the buttons to the stop ( only once), holding the inhaler in a vertical position. NOTE: at this moment, the capsule may break and small pieces may enter the mouth or throat. Swallowing them is not harmful. The likelihood of such an event is minimal if the capsule is not pierced more than once, storage conditions are maintained, and the capsule is removed from the blister pack immediately before use (see point 3).
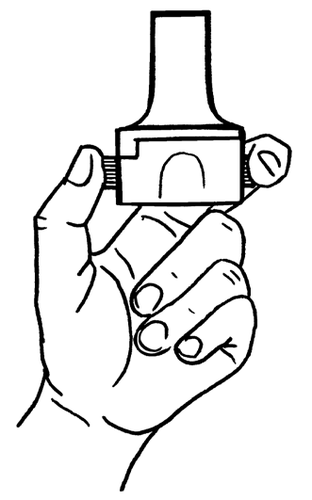
- 6. Take a deep breath out.
- 7. Place the mouthpiece in your mouth and slightly tilt your head back. Close your lips around the mouthpiece and take one quick and even breath in. As the capsule rotates in the inhaler chamber and the powder is dispersed, a characteristic sound (rattling) should be heard. If this sound does not occur, it may mean that the capsule is stuck in the compartment. You should then open the inhaler and remove the capsule from the compartment by lifting it. DO NOT try to remove the capsule by pressing the buttons multiple times.
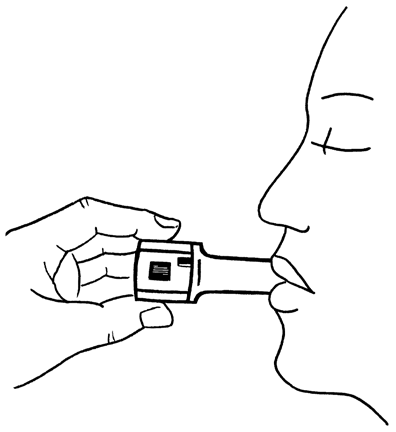
- 8. After hearing the characteristic sound (rattling), you should hold your breath for as long as possible without discomfort and then remove the inhaler from your mouth. Take a breath out. Open the inhaler and check if there is still powder in the capsule. If powder remains in the capsule, repeat the steps described in points 6 to 8.
- 9. After use, open the inhaler, remove the empty capsule, close the mouthpiece, and put the cap back on.
Cleaning the inhaler:
To remove powder residue, you should wipe the mouthpiece and capsule compartment with a dry cloth or a clean, soft brush.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAdamed Pharma S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FlutixonDosage form: Powder, 250 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Powder, 500 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Powder, 50 mcg/dose inh.Active substance: fluticasonePrescription required
Alternatives to Flutixon in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Flutixon in Украина
Alternative to Flutixon in Испания
Online doctors for Flutixon
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Flutixon – subject to medical assessment and local rules.