Flixotide

Ask a doctor about a prescription for Flixotide

How to use Flixotide
Leaflet accompanying the packaging: patient information
Flixotide, 250 g/inhalation dose, inhalation aerosol, suspension
Fluticasone propionate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What Flixotide is and what it is used for
- 2. Important information before using Flixotide
- 3. How to use Flixotide
- 4. Possible side effects
- 5. How to store Flixotide
- 6. Contents of the packaging and other information
1. What Flixotide is and what it is used for
Flixotide, 250 µg/inhalation dose, inhalation aerosol, suspension belongs to a group of medicines used in obstructive airway diseases. The active substance of the medicine, fluticasone propionate, is a corticosteroid with local anti-inflammatory action in the lungs.
The medicine is indicated in:
- preventing asthma attacks in adults and children over 16 years of age:
- mild asthma - in patients who require daily symptomatic treatment with bronchodilators;
- moderate asthma - unstable or worsening asthma, despite regular use of preventive asthma medications or only bronchodilators;
- severe asthma - in patients with severe chronic asthma and requiring oral steroids to control asthma symptoms. Starting fluticasone propionate treatment in many people allows for a reduction in doses or complete discontinuation of oral steroids;
- treatment of chronic obstructive pulmonary disease (COPD).Fluticasone propionate is indicated for the treatment of COPD, in combination with a long-acting beta-agonist, such as salmeterol.
The inhalation aerosol, suspension, Flixotide only with a strength of 250 µg/inhalation dose, is suitable for use in this indication.
2. Important information before using Flixotide
When not to use Flixotide
- if the patient is allergic to fluticasone propionate or to 1,1,1,2-tetrafluoroethane (HFA 134a),
a helper substance in Flixotide.
Warnings and precautions
If asthma symptoms worsen or asthma control deteriorates, i.e.if wheezing worsens or it is necessary to use a larger number of inhalations of a rapid-acting inhalation medication as needed, the patient should continue using the medicine and contact their doctor immediately, who will assess the patient's condition and recommend appropriate treatment.
Flixotide should not be used to interrupt a sudden asthma attack. For this purpose, a rapid-acting bronchodilator (e.g., salbutamol) should be used, which the patient should always carry with them. Care should be taken not to confuse Flixotide with an inhalation medication used as needed.
The doctor should periodically check the patient's inhalation technique to ensure that the release of the medicine from the inhaler is properly synchronized with the performance of a deep, calm breath. During inhalation, the patient should sit or stand. The inhaler is designed for use in an upright position.
With long-term use of Flixotide, suppression of the natural production of steroid hormones by the adrenal glands may occur. This may cause a decrease in bone mass, cataracts, glaucoma, weight gain, rounding of the face (moon face), high blood pressure, slowing of growth in children and adolescents. The doctor will regularly check for any of these side effects and ensure that the patient is using the smallest dose of Flixotide that provides effective control of asthma and COPD.
During treatment with fluticasone propionate at recommended doses, adrenal cortex function usually remains normal. Nevertheless, in people previously treated with oral steroids, symptoms of impaired adrenal cortex function may occur. Long-term treatment with high doses of inhaled steroids may cause suppression of adrenal cortex function. Children and adolescents under 16 years of age taking large doses (usually ≥1000 micrograms per day) of fluticasone propionate are at particular risk. Very rare side effects may occur when taking large doses of Flixotide for a long time or when suddenly stopping treatment or reducing the dose. Side effects may also occur in the case of infections or during periods of severe stress (e.g., accident or surgery). The symptoms are not usually characteristic and may include: abdominal pain, fatigue, loss of appetite, nausea and vomiting, weight loss, headache, confusion, low blood pressure, decreased blood glucose levels, and seizures. To prevent these symptoms, the doctor may prescribe additional corticosteroids for use at that time.
Due to the possibility of impaired adrenal cortex function, patients who are switching from oral steroid medications to inhaled fluticasone propionate should be under special care, and adrenal cortex function should be monitored.
After introducing fluticasone propionate to treatment, the reduction of oral steroid doses should be gradual, and patients should carry a "steroid card" informing them of the need for additional oral steroids in stressful situations.
Replacing oral steroids with inhaled medications may reveal allergic symptoms, such as: allergic rhinitis or rash, which were previously treated with oral steroids. The doctor will recommend appropriate treatment.
There have been reports of an increased number of cases of pneumonia in clinical trials in patients with chronic obstructive pulmonary disease (COPD) receiving fluticasone propionate at a dose of 500 micrograms (see section 4). The doctor should monitor patients with COPD for the development of pneumonia, as the clinical symptoms of pneumonia and COPD exacerbation may often overlap.
Very rare cases of increased blood glucose levels have been reported (see section 4), and the doctor should take this into account when prescribing Flixotide to patients with a history of diabetes.
Do not stop treatment with fluticasone propionate suddenly.
If the patient is currently being treated or has been treated for tuberculosis, they should inform their doctor.
If the patient experiences blurred vision or other vision disturbances that may be caused by cataracts or glaucoma, they should contact their doctor.
Flixotide and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription.
It is especially important to tell your doctor about any of the following medicines you are currently taking or have recently taken:
- corticosteroids in tablets or injections
- ritonavir or medicines containing cobicistat, used to treat HIV
- ketoconazole or itraconazole, used to treat fungal infections.
The doctor will assess whether Flixotide can be used with these medicines. Some of them may enhance the effect of Flixotide, and the doctor may want to monitor the patient's condition closely when taking such medicines (including some HIV medicines: ritonavir, cobicistat).
Only medicines recommended by the doctor should be used with Flixotide.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine. The doctor will assess whether the patient can take Flixotide during this period.
Driving and using machines
It is unlikely that side effects associated with the use of Flixotide will affect the ability to drive or operate machinery.
3. How to use Flixotide
This medicine should always be used as directed by the doctor. Do not take a higher dose than recommended. If in doubt, consult your doctor.
Flixotide in the form of an inhalation aerosol is intended exclusively for inhalation use.
The doctor will adjust the dose of the medicine according to the individual patient's response to treatment and determine the smallest dose that provides effective control of symptoms.
To facilitate the use of the medicine and prevent potential side effects in the mouth and throat, patients treated with inhaled steroids, especially those who have difficulty coordinating inhalation with the release of the medicine from the inhaler (e.g., children and elderly patients), are advised to use a spacer.
It is very important to use Flixotide every day, until the doctor recommends otherwise. The therapeutic effect occurs within 4 to 7 days.
Asthma: Adults and children over 16 years of age
From 100 µg to 1000 µg twice a day.
The initial dose of the medicine depends on the severity of the disease:
- mild asthma: from 100 µg to 250 µg twice a day;
- moderate asthma: from 250 µg to 500 µg twice a day;
- severe asthma: from 500 µg to 1000 µg twice a day.
Chronic obstructive pulmonary disease (COPD): Adults
500 µg twice a day, which corresponds to 2 doses of Flixotide, inhalation aerosol, suspension 250 µg/inhalation dose, in combination with a long-acting beta-agonist, such as salmeterol.
The inhalation aerosol, suspension, Flixotide only with a strength of 250 µg/inhalation dose, is suitable for use in this indication.
The medicine must be used daily to achieve optimal benefit, which may take from three to six months.
If there is no improvement, the patient should consult their doctor for re-evaluation.
Special patient groups
There is no need to change the dosage in elderly patients or patients with impaired renal or hepatic function.
Instructions for using the inhaler
- The doctor, nurse, or pharmacist should instruct the patient on how to use the inhaler properly. They should periodically check that the patient is using the inhaler correctly. Not using Flixotide as directed by the doctor or not using the inhaler correctly may cause the medicine to not produce the expected improvement in asthma or COPD.
- The medicine is contained in a pressurized container, in a plastic casing with a mouthpiece.
Checking the inhaler
- 1. Before first use, check that the inhaler is working. Remove the cap from the mouthpiece of the inhaler by gently pressing the sides of the cap with your thumb and index finger.
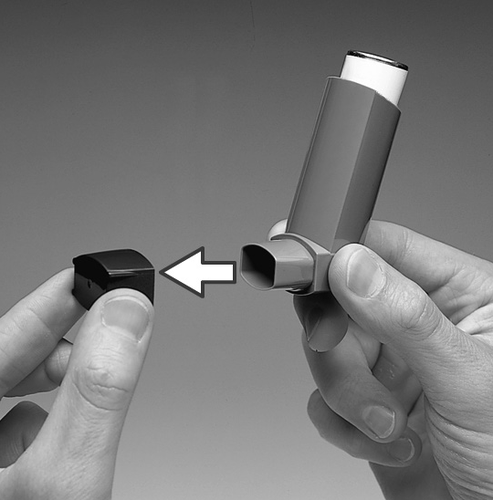
- 2. To ensure that the inhaler is working, shake it vigorously, point the mouthpiece away from you, and release a dose of the medicine into the air. If the inhaler has not been used for a week or longer, remove the mouthpiece cap, shake it vigorously, and release two doses of the medicine into the air.
Using the inhaler
It is essential to start slow breathing, as slow as possible, before using the inhaler.
- 1. Inhale while standing or sitting. The inhaler is designed for use in an upright position.
- 2. Remove the cap from the mouthpiece of the inhaler (as shown in figure 1). Check the mouthpiece outside and inside to ensure it is clean and free of foreign objects.
- 3. Shake the inhaler 4 or 5 times to ensure that any foreign objects are removed and the contents of the inhaler are well mixed.

It is essential not to rush through the actions described in points 4-7.
- 4. Hold the inhaler upright with your fingers, with your thumb on the base of the inhaler, under the mouthpiece. Take a deep breath out, as deep as possible.

- 5. Put the mouthpiece in your mouth and seal it with your lips. Do not bite the mouthpiece.

- 6. Immediately after starting to inhale through your mouth, press the inhaler to release the Flixotide medicine, and then continue with a calm, deep inhalation.

- 7. Hold your breath, remove the inhaler from your mouth, and take your finger off the inhaler base. Hold your breath for a few seconds or as long as is comfortable, then breathe out slowly.
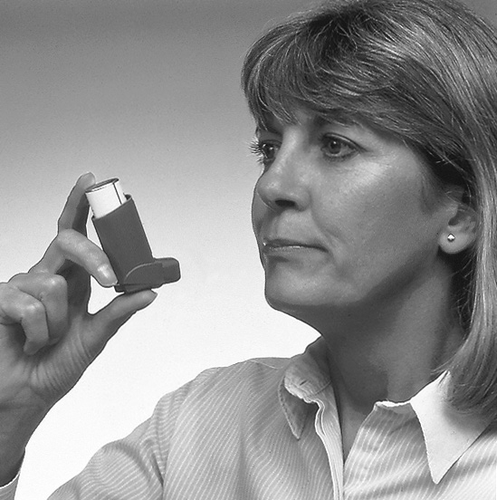
- 8. If the doctor has recommended two inhalations of the medicine, wait about half a minute before repeating the actions described in points 3-7.
- 9. Rinsing the mouth with water and spitting it out after inhalation helps prevent hoarseness and thrush.
- 10. After inhalation, always replace the cap on the mouthpiece to prevent dust from entering it. Replace the cap by clicking it into place. If you do not hear a click when replacing the cap, remove it, turn it over, and try to replace it again. Do not use too much force.
Cleaning the inhaler
To prevent the inhaler from clogging, it should be cleaned at least once a week. To clean the inhaler:
- Remove the cap from the mouthpiece.
- Do not remove the metal container from the plastic casing during cleaning or at any other time.
- Clean the mouthpiece inside and outside and the plastic casing outside with a dry cloth or tissue.
- Replace the cap on the mouthpiece. When the cap is properly replaced, you will hear a click. If you do not hear a click when replacing the cap, remove it, turn it over, and try to replace it again. Do not use too much force.
- Do not immerse the metal container in water.
Using a higher than recommended dose of Flixotide
In the event of using a higher than recommended dose of Flixotide, consult your doctor or pharmacist immediately for advice.
It is essential to use the recommended doses of the medicine. Do not increase or decrease the dose without consulting your doctor.
Taking higher than recommended doses of fluticasone propionate may cause temporary suppression of adrenal cortex function.
Long-term use of higher than recommended doses of fluticasone propionate may lead to adrenal cortex insufficiency.
Missing a dose of Flixotide
It is crucial to take the recommended dose of the medicine every day to ensure the greatest effectiveness of treatment.
If a dose is missed, take the medicine as soon as possible. Continue treatment as before.
Do not take a double dose to make up for a missed dose.
Stopping treatment with Flixotide
It is very important to take Flixotide every day until the doctor recommends stopping treatment. Do not stop taking Flixotide suddenly, as the symptoms of the disease may worsen, and hormonal disorders may occur in the body.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Flixotide can cause side effects, although not everybody gets them.
The following side effects have been observed in patients taking Flixotide:
Allergic reactions: seek medical help immediately
Allergic reactionsto Flixotide, which occur uncommonly (may affect 1 to 10 in 1000 patients taking the medicine). They are characterized by symptoms such as:
- Hives (urticaria) or redness.
Allergic reactionsto Flixotide, which occur very rarely (may affect less than 1 in 10,000 patients taking the medicine), and in a small number of patients, these reactions may develop into a serious, life-threatening condition if not treated. They are characterized by symptoms such as:
- Swelling (mainly of the face, lips, tongue, or throat), which may cause difficulty swallowing or breathing.
- Sudden difficulty breathing or a sudden worsening of wheezing (bronchospasm).
- Sudden feeling of weakness or dizziness (which may lead to falls or loss of consciousness).
If such symptoms occur, stop using the medicine and seek medical help immediately.
Pneumonia (lung infection) in patients with chronic obstructive pulmonary disease (COPD) (common side effect)
Tell your doctorif any of the following symptoms occur while using Flixotide - they may be symptoms of a lung infection:
- Fever or chills.
- Increased production of mucus, change in mucus color.
- Worsening cough or increased breathing difficulties.
Other side effects:
Very common side effects(may affect more than 1 in 10 patients taking the medicine):
- Thrush (painful, creamy-white patches) in the mouth and throat, difficulty swallowing. Rinsing the mouth with water and spitting it out after each inhalation may be helpful. The doctor may also recommend an antifungal medicine to treat thrush.
Common side effects(may affect 1 to 10 in 100 patients taking the medicine):
- Hoarseness, loss of voice. Rinsing the mouth with water and spitting it out after each inhalation may be helpful.
- Pneumonia has been reported in patients with COPD - see above.
- Easy bruising.
Rare side effects(may affect 1 to 10 in 10,000 patients taking the medicine):
- Thrush of the esophagus.
Very rare side effects(may affect less than 1 in 10,000 patients taking the medicine):
- Flixotide may suppress the normal production of steroid hormones in the body, particularly when taking large doses of the medicine for a long time. Symptoms include slowing of growth in children and adolescents, decreased bone mass, cataracts, glaucoma, weight gain, rounding of the face (moon face), high blood pressure, and an increase in blood glucose levels.
- Increased blood glucose levels (hyperglycemia). In patients with diabetes, it may be necessary to monitor blood glucose levels more frequently and adjust the dose of antidiabetic medicines.
- Joint pain.
- Indigestion.
- Anxiety, sleep disturbances, and changes in behavior, including hyperactivity and irritability. The occurrence of these side effects is more likely in children.
Side effects with unknown frequency(frequency cannot be estimated from the available data):
- Depression and aggression. The occurrence of these side effects is more likely in children.
- Nosebleeds.
- Blurred vision.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Flixotide
Keep the medicine out of the sight and reach of children.
Store at a temperature not exceeding 30 °C.
Do not freeze.
The container contains a suspension under pressure. Do not expose to temperatures above 50 ° C, protect from direct sunlight. Do not pierce, damage, burn, or throw away the container, even if it seems empty.
As with most inhaled medicines in pressurized containers, the effectiveness of this medicine may be reduced if the inhaler is cold.
After inhalation, replace the cap on the mouthpiece. Do not force the cap on.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month stated.
The batch number of the medicine is stated on the packaging: Lot.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Flixotide contains
- The active substance of the medicine is fluticasone propionate (micronized). One dose contains 250 µg (micrograms) of fluticasone propionate, micronized.
- The other ingredient is 1,1,1,2-tetrafluoroethane (HFA 134a). The propellant gas (HFA 134a) does not contain freons.
This medicine contains fluorinated greenhouse gases.
60 doses
Each inhaler contains 8 g of HFC-134a (also known as 1,1,1,2-tetrafluoroethane or HFA 134a), which corresponds to 0.0114 tons of CO2 equivalent (GWP = 1430).
120 doses
Each inhaler contains 12 g of HFC-134a (also known as 1,1,1,2-tetrafluoroethane or HFA 134a), which corresponds to 0.0172 tons of CO2 equivalent (GWP = 1430).
What Flixotide looks like and contents of the pack
Inhalation aerosol, suspension - 60 or 120 doses of the medicine in an aluminum container under pressure, closed with a metering valve, in a cardboard box.
Manufacturer:
Glaxo Wellcome Production
Zone Industrielle No. 2
23, rue Lavoisier
27000, Evreux
France
Glaxo Wellcome S.A.
Avenida de Extremadura 3
09400 Aranda de Duero
Burgos
Spain
To obtain more detailed information, contact the representative of the marketing authorization holder:
GSK Services Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warsaw
tel. (22) 576-90-00
{Logo of the marketing authorization holder}
Date of last revision of the leaflet: January 2025
Marketing authorization holder:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
D24 YK11
Ireland
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlaxo Wellcome Production Glaxo Wellcome S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FlixotideDosage form: Powder, 250 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Powder, 500 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Powder, 50 mcg/dose inh.Active substance: fluticasonePrescription required
Alternatives to Flixotide in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Flixotide in Ukraine
Alternative to Flixotide in Spain
Online doctors for Flixotide
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Flixotide – subject to medical assessment and local rules.