
How to use Fixapost
Leaflet accompanying the packaging: patient information
Fixapost, 50 micrograms/ml + 5 mg/ml, eye drops, solution in a container
single-dose
Latanoprost+ Timolol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Fixapost and what is it used for
- 2. Important information before using Fixapost
- 3. How to use Fixapost
- 4. Possible side effects
- 5. How to store Fixapost
- 6. Contents of the packaging and other information
1. What is Fixapost and what is it used for
Fixapost contains two active substances: latanoprost and timolol. Latanoprost belongs to a group of medicines known as prostaglandin analogues. Timolol belongs to a group of medicines called beta-adrenergic blockers.
Latanoprost works by increasing the natural outflow of aqueous fluid from the eye into the bloodstream.
Timolol reduces the production of aqueous fluid in the eye.
Fixapost is used to reduce pressure in the eye, if the patient has open-angle glaucoma or increased intraocular pressure. Both of these conditions (states) are associated with increased pressure inside the eyeball and can affect vision. The attending physician prescribes Fixapost to the patient if other medicines are not effective enough.
2. Important information before using Fixapost
Fixapost can be used by adult men and women (including the elderly), but it is not recommended for people under 18 years of age.
When not to use Fixapost:
- if the patient is allergic (hypersensitive) to latanoprost, timolol, beta-adrenergic blockers, or any of the other ingredients of this medicine (listed in section 6),
- if the patient currently has or has had respiratory disorders, such as asthma, severe chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, and/or prolonged coughing),
- if the patient has serious heart problems or arrhythmias (irregular heartbeat).
Warnings and precautions
Before starting to use Fixapost, the patient should discuss with their doctor, pharmacist, or nurse if any of the following situations apply to them currently or have applied in the past:
- ischemic heart disease (symptoms may include chest pain or tightness, shortness of breath, choking), heart failure, low blood pressure,
- arrhythmias, such as slow heartbeat,
- breathing problems, asthma, chronic obstructive pulmonary disease,
- if the patient has a condition related to poor blood circulation (such as Raynaud's disease or syndrome),
- diabetes, as timolol may mask the subjective and objective symptoms associated with low blood sugar levels,
- hyperthyroidism, as timolol may mask the subjective and objective symptoms of this condition,
- if the patient is scheduled to undergo any eye surgery (including cataract surgery) or has had such surgery in the past,
- if the patient suffers from eye diseases (such as eye pain, eye irritation, eye inflammation, or blurred vision),
- if the patient has dry eye syndrome,
- if the patient wears contact lenses. Fixapost can still be used, but the patient should follow the instructions for contact lens wearers in section 3,
- if the patient suffers from variant angina (especially Prinzmetal's angina),
- if the patient has had severe allergic reactions, which usually require hospital treatment,
- if the patient has had or currently has a viral eye infection caused by the herpes simplex virus (HSV).
Before surgery, the patient should inform their doctor about the use of Fixapost, as timolol may affect the action of certain medicines used during anesthesia.
Fixapost and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including eye drops and over-the-counter medicines.
Certain medicines may affect the action of Fixapost, and Fixapost may affect the action of other medicines, including other eye drops used to treat glaucoma. The patient should tell their doctor about the use of or intention to use medicines that lower blood pressure, heart medicines, or anti-diabetic medicines.
In particular, the patient should discuss with their doctor or pharmacist if they are taking any of the following medicines:
- prostaglandins, analogues, or derivatives of prostaglandins,
- beta-adrenergic blockers,
- epinephrine,
- medicines used to lower blood pressure, such as oral calcium channel blockers, guanethidine, anti-arrhythmic medicines, digitalis glycosides, or parasympathomimetics,
- quinidine (a medicine used to treat heart conditions and certain types of malaria),
- antidepressant medicines known as fluoxetine and paroxetine.
Fixapost with food and drink
Eating meals, food, and drink do not affect the time and method of using Fixapost.
Pregnancy, breastfeeding, and fertility
Fixapost should not be usedduring pregnancy or breastfeeding.
Fixapost may pass into breast milk.
In animal studies, it has been found that latanoprost and timolol do not affect fertility in women or men.
If the patient is pregnant, thinks she may be pregnant, or plans to have a child, she should consult her doctor before using this medicine.
Driving and using machines
Using Fixapost may cause temporary vision disturbances. In such cases, the patient should not drive vehicles, use tools, or operate machines until the disturbances have resolved.
Fixapost contains macrogolglycerol hydroxystearate(derived from castor oil) which may cause skin reactions.
3. How to use Fixapost
This medicine should always be used as directed by the doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
The recommended dose for adults (including the elderly) is one drop into the affected eye(s) once daily.
Fixapost should not be used more frequently than once daily, as more frequent use may reduce the effectiveness of therapy.
Fixapost should be used as directed by the doctor until the doctor decides to stop the treatment.
The doctor may recommend more frequent checks of the patient's heart and circulatory system during the use of Fixapost.
Contact lens wearers
Before administering Fixapost, the patient should remove their contact lenses. After using the medicine, they should wait 15 minutes before reinserting the lenses.
Method of administration
This medicine is intended for administration into the eye.
To apply the drops, the patient should follow the instructions below:
- 1. Wash hands and take a comfortable standing or sitting position.
- 2. Open the sachet containing 5 single-dose containers. Record the date of first opening on the sachet.
- 3. Break off one single-dose container from the strip.
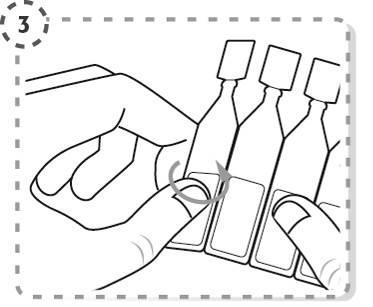
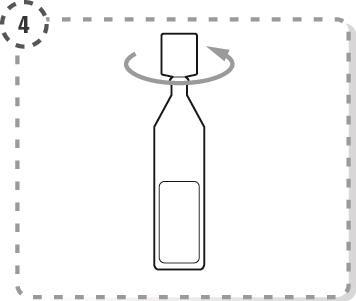
- 4. Unscrew the top of the single-dose container, as shown in the diagram. Do not touch the tip of the container after opening.
- 5. Gently pull down the lower eyelid of the affected eye.
- 6. Place the tip of the single-dose container close to the eye, without touching the surface of the eye.
- 7. Gently squeeze the single-dose container to release one drop into the eye, then release the lower eyelid.
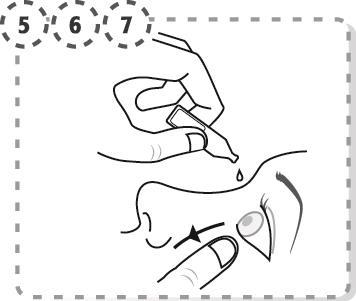
- 8. Press the inner corner of the eye at the base of the nose with a finger. Hold for 2 minutes, with eyes closed.
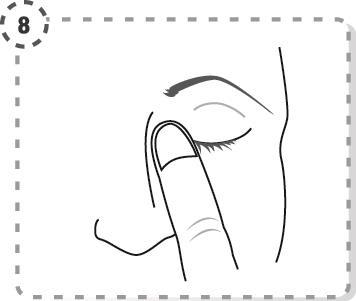
- 9. Repeat the steps for the second eye, if the doctor has recommended it. Each single-dose container contains enough solution to treat both eyes.
- 10. Discard the single-dose container after use. Do not store it for reuse. Since the sterility of a single single-dose container cannot be maintained after opening, a new container must be opened before each use.
- 11. Place the unopened single-dose containers back in the sachet. Store the opened sachet in the carton. After the first opening of the sachet: the shelf life of the unopened single-dose containers is 1 month.
Using Fixapost with other eye drops
The patient should wait at least 5 minutes between using Fixapost and administering other eye drops.
Using a higher dose of Fixapost than recommended
Using too many drops in the eye may cause mild eye irritation, tearing, and redness. These symptoms should resolve, but if they bother the patient, they should consult their doctor.
Swallowing Fixapost drops
In case of accidental ingestion of Fixapost, the patient should contact their doctor for advice. Swallowing a large amount of Fixapost drops may cause nausea, stomach pain, feeling of tiredness, flushing of the face, dizziness, and sweating.
Missing a dose of Fixapost
The patient should continue with the scheduled dose at the usual time. They should not use a double dose to make up for a missed dose. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Fixapost can cause side effects, although not everybody gets them.
It is usually possible to continue using the drops, unless the side effects are severe. If in doubt, the patient should consult their doctor or pharmacist. The patient should not stop using Fixapost without consulting their doctor.
The following are known side effects associated with the use of Fixapost. The most important possible side effect is a gradual, permanent change in eye color. There is also a possibility of serious changes in heart function. If the patient experiences changes in heart rhythm or function, they should consult their doctor and inform them about the use of Fixapost.
The following side effects have been observed during the use of Fixapost:
Very common (may affect more than 1 in 10 people):
- Gradual change in eye color due to an increase in the amount of brown pigment in the colored part of the eye, i.e., the iris. This effect is more frequently observed in patients with mixed-color irises (blue-brown, gray-brown, yellow-brown, or green-brown) than in patients with uniform eye color (blue, gray, green, or brown). The change in eye color may take years to develop. This change may be permanent and may be more noticeable if Fixapost is administered to only one eye. No accompanying symptoms have been observed with the change in eye color. These changes do not worsen after stopping the use of Fixapost.
Common (may affect less than 1 in 10 people):
- Eye irritation (feeling of burning, grittiness, itching, stinging, or foreign body sensation in the eye) and eye pain.
Uncommon (may affect less than 1 in 100 people):
- Headache,
- Eye redness, eye infection (conjunctivitis), blurred vision, tearing, eyelid inflammation, irritation, or damage to the surface of the eye,
- Skin rash or itching,
- Nausea, vomiting.
Other side effects
Like other eye medicines, Fixapost (latanoprost and timolol) is absorbed into the bloodstream.
The frequency of side effects after using eye drops is lower than when taking medicines orally or by injection.
Although the following additional side effects have not been observed with Fixapost, they may occur during therapy, as they have been reported with the substances contained in Fixapost (latanoprost and timolol). The following side effects include reactions observed during the treatment of eye diseases with beta-adrenergic blockers (e.g., timolol):
- Development of viral eye infection caused by the herpes simplex virus (HSV)
- General allergic reactions, including angioedema, which may occur in the face and limbs and may affect breathing and swallowing, urticaria, or pruritic rash, localized and generalized rash, itching, severe, life-threatening allergic reaction.
- Low blood sugar levels.
- Dizziness
- Difficulty sleeping (insomnia), depression, nightmares, memory loss, hallucinations.
- Fainting, stroke, decreased blood flow to the brain, worsening of myasthenia symptoms (muscle disorders), unusual sensations, such as tingling and prickling, headache.
- Edema in the back of the eye (macular edema), cyst in the colored part of the eye (iris cyst), sensitivity to light (photophobia), appearance of sunken eyes (deepening of the eyelid sulcus).
- Symptoms of eye irritation (e.g., burning, stinging, itching, tearing, redness), eyelid inflammation, keratitis, blurred vision, and detachment of the layer under the retina, which can cause vision disturbances, decreased corneal sensitivity, dry eye, erosion (ulceration) of the cornea (damage to the front layer of the eyeball), drooping of the upper eyelid (causing the eye to be half-closed), double vision.
- Darkening of the skin around the eyes, changes in the appearance of eyelashes and fine hairs around the eye (increased number, length, thickness, and darkening), changes in the direction of eyelash growth, swelling around the eye, swelling of the colored part of the eye (uveitis), scarring of the surface of the eye.
- Ringing/buzzing in the ears (tinnitus).
- Angina, worsening of angina pectoris in patients with heart disease.
- Slow heart rate, chest pain, palpitations (feeling of heartbeat), swelling (fluid accumulation), changes in heart rhythm or rate, congestive heart failure (heart disease with shortness of breath and swelling of the feet and ankles due to fluid accumulation), a type of heart rhythm disorder, heart attack, heart failure.
- Low blood pressure, poor blood circulation, causing numbness and pallor of the fingers and toes, cold hands and feet.
- Shallow breathing (shortness of breath), narrowing of the airways in the lungs (mainly in patients with pre-existing lung disease), difficulty breathing, coughing, asthma, worsening of asthma.
- Taste disturbances, nausea, indigestion, diarrhea, dry mouth, stomach pain, vomiting.
- Hair loss, skin rash with a white-silver appearance (psoriasis-like or worsening of psoriasis, skin rash).
- Joint pain, muscle pain not caused by physical exertion, muscle weakness, fatigue.
- Sexual function disorders, decreased libido.
Reporting side effects
If side effects occur, including those not listed in the leaflet, the patient should tell their doctor or pharmacist.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw
phone: +48 22 49 21 301, fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Fixapost
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton, sachet, and single-dose container. The expiry date refers to the last day of the month.
There are no special storage instructions for the temperature.
After the first opening of the sachet: the shelf life of the unopened single-dose containers is 1 month.
The patient should record the date of first opening on the sachet.
After the first opening of the single-dose container: use immediately and discard the single-dose container after use.
Store the single-dose containers in the sachet to protect them from light.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer use. This will help protect the environment.
6. Contents of the packaging and other information
What Fixapost contains
The active substances are latanoprost 50 micrograms/ml and timolol (as timolol maleate) 5 mg/ml.
The other ingredients are: macrogolglycerol hydroxystearate, sorbitol, macrogol, carbomer, disodium edetate, sodium hydroxide (for pH adjustment), water for injections.
What Fixapost looks like and contents of the pack
This medicinal product is available as eye drops, solution in a single-dose container. The solution is slightly yellow and opalescent, does not contain preservatives, and is practically free from visible particles. It is available in single-dose containers, packaged 5 in a sachet. Each single-dose container contains 0.2 ml of the medicine.
The packaging contains 30 (6 x 5) or 90 (18 x 5) single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Laboratoires THEA
12 rue Louis Blériot
63017 Clermont-Ferrand Cedex 2
France
Manufacturer
EXCELVISION
27, rue de la Lombardière
07100 Annonay
France
Laboratoires THEA
12 rue Louis Blériot
63017 Clermont-Ferrand Cedex 2
France
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Czech Republic, France, Ireland, Italy, Poland, Slovakia, United Kingdom ............................................................................................................. Fixapost
Belgium, Bulgaria, Cyprus, Germany, Greece, Spain, Luxembourg, Netherlands ............... Fixaprost
Denmark, Estonia, Finland, Iceland, Lithuania, Latvia, Norway, Sweden ....................... Fixopost
Croatia, Slovenia ..................................................................................................... Fixalpost
Romania ........................................................................................................................ Fixanpost
Portugal ...................................................................................................................... Monoprost Duo
Date of last revision of the leaflet: 18-03-2022
Detailed information on this medicine is available on the website www.urpl.gov.pl
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterExcelvision Laboratoires THEA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FixapostDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Fixapost in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Fixapost in España
Alternative to Fixapost in Ucrania
Online doctors for Fixapost
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Fixapost – subject to medical assessment and local rules.






