
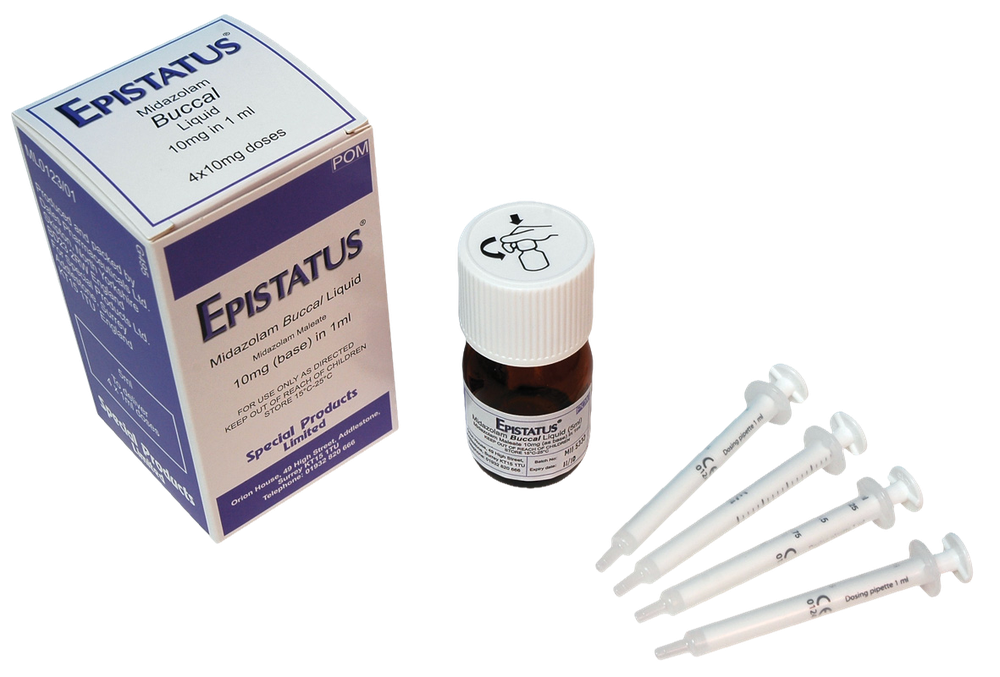
Epistatus

Ask a doctor about a prescription for Epistatus

How to use Epistatus
Leaflet accompanying the packaging: information for the user
Epistatus, 2.5 mg, solution for oral use
Epistatus, 5 mg, solution for oral use
Epistatus, 7.5 mg, solution for oral use
Epistatus, 10 mg, solution for oral use
Midazolam
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Epistatus and what is it used for
- 2. Important information before using Epistatus
- 3. How to use Epistatus
- 4. Possible side effects
- 5. How to store Epistatus
- 6. Contents of the packaging and other information
1. What is Epistatus and what is it used for
Epistatus contains the active substance midazolam, which belongs to a group of medicines called benzodiazepines.
Epistatus is used to stop prolonged, acute seizure attacks in infants, young children, children, and adolescents from 3 months to less than 18 years of age.
In the case of infants from 3 to 6 months of age, this medicine should be used in a hospital setting where monitoring and access to resuscitation equipment are possible (see section Warnings and precautions).
This medicine may only be administered by parents or caregivers when the child has been diagnosed with epilepsy. The doctor treating the patient should provide the parents or caregivers with instructions on how to administer Epistatus and what to do if the seizure does not stop (see also section "How to use Epistatus").
2. Important information before using Epistatus
When not to use Epistatus
- if the patient is allergic to midazolam, other benzodiazepines (such as diazepam or nitrazepam), or any of the other ingredients of this medicine (listed in section 6).
- if the patient has a disease called myasthenia (which causes muscle weakness).
- if the patient has significant breathing difficulties (Epistatus may worsen breathing difficulties).
- if the patient has sleep apnea syndrome (which causes frequent breaks in breathing during sleep).
- if the patient has severe liver function disorders.
Warnings and precautions
Before starting to use Epistatus, discuss with your doctor or pharmacist if the patient:
- has a lung disease that causes breathing problems, as this medicine may worsen breathing;
- has kidney, liver, or heart problems;
- takes other sedative medicines and feels very weak, tired, and lacks energy, as this medicine affects the central nervous system (CNS);
- regularly consumes large amounts of alcohol or has had problems with alcohol abuse in the past (see section "Epistatus contains ethanol (alcohol)");
- regularly uses non-prescription medicines or has had problems with drug abuse in the past.
This medicine may affect the patient's memory after administration (temporary memory loss).
Parents or caregivers should closely monitor patients after administering this medicine. See also section 4 ("Possible side effects").
Because younger children cannot be ruled out for delayed severe breathing problems (such as slower or weaker than expected breathing), infants from 3 months to less than 6 months of age should only be treated in a hospital setting where vital functions can be monitored and resuscitation equipment is available.
In case of doubts about any of the above situations, consult a doctor or pharmacist before administering this medicine.
Children and adolescents
This medicine must not be given to children under 3 months of age, as there is not enough information for this age group.
Epistatus and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take. In case of any doubts about whether any medicine taken by the patient may have a negative impact on the use of Epistatus, consult a doctor or pharmacist.
This is very important, as taking more than one medicine at the same time can enhance or weaken the effect of these medicines.
The effect of Epistatus may be enhanced by medicines such as:
- antiepileptic medicines (used to treat epilepsy), e.g., phenytoin;
- antibiotics, e.g., erythromycin, clarithromycin;
- antifungal medicines, e.g., ketoconazole, voriconazole, fluconazole, itraconazole, posaconazole;
- medicines used to treat stomach and duodenal ulcers, e.g., cimetidine, ranitidine, and omeprazole;
- medicines used to treat high blood pressure, e.g., diltiazem, verapamil;
- certain medicines used to treat HIV and AIDS, e.g., saquinavir, lopinavir + ritonavir;
- opioid painkillers (very strong painkillers), e.g., fentanyl;
- medicines used to reduce the amount of fat in the blood, e.g., atorvastatin;
- medicines used to treat nausea, e.g., nabilone;
- sleeping medicines (medicines that cause sleep);
- sedative antidepressants (medicines used to treat depression that cause sleepiness);
- sedative medicines (medicines that cause relaxation);
- anesthetics (used to relieve pain);
- antihistamine medicines (used to treat allergies).
The effect of Epistatus may be weakened by medicines such as:
- rifampicin (used to treat tuberculosis);
- xanthines (used to treat asthma);
- St. John's Wort (a herbal medicine). Patients taking Epistatus should avoid using it.
Epistatus may also enhance the effect of certain muscle relaxants, e.g., baclofen (causing increased sleepiness). This medicine may also inhibit the effect of certain other medicines, e.g., levodopa (a medicine used to treat Parkinson's disease).
Taking Epistatus and opioids (contained in very strong painkillers, replacement therapy medicines, and some cough medicines) at the same time increases the risk of sleepiness, breathing difficulties (respiratory depression), coma, and can also be life-threatening.
Therefore, concurrent use should only be considered when other treatment options are not possible.
However, if a doctor prescribes Epistatus with opioids, the dose of the medicines and the duration of concurrent treatment should be limited.
Tell your doctor about all opioid medicines being taken and strictly follow the doctor's instructions regarding dosage. Consider informing friends or relatives so they know the above-mentioned symptoms. Contact a doctor if these symptoms occur.
Epistatus contains a small amount of alcohol and should not be taken at the same time as disulfiram.
Consult a doctor or pharmacist for information on which medicines to avoid during treatment with Epistatus.
Procedures
If the patient is to be given inhaled anesthesia (products administered during breathing) during a surgical procedure or dental treatment, it is essential to inform the doctor or dentist that the patient is taking Epistatus.
Epistatus with food, drink, and alcohol
While taking Epistatus, do not drink alcohol. Alcohol may enhance the sedative effect of Epistatus and cause increased sleepiness.
While taking Epistatus, do not drink grapefruit juice. Grapefruit juice may enhance the sedative effect of Epistatus and cause increased sleepiness.
Pregnancy, breastfeeding, and fertility
Pregnancy
If the patient is pregnant, thinks she may be pregnant, or plans to have a child, she should consult a doctor before using this medicine.
Midazolam may be used during pregnancy if necessary. Frequent administration of Epistatus in the last 3 months of pregnancy or during delivery may cause problems in the baby, such as: heart rhythm disturbances, hypothermia (low body temperature), weak sucking reflex, breathing difficulties, and weak muscle tone.
Breastfeeding
Tell your doctor if the patient is breastfeeding. Although small amounts of Epistatus may pass into breast milk, breastfeeding may not need to be discontinued. The doctor will decide whether the patient should temporarily stop breastfeeding after administering Epistatus.
Driving and using machines
Epistatus has a significant impact on the ability to drive and use machines.
Epistatus may cause sleepiness, forgetfulness, or weaken concentration and coordination in the patient. Such effects may disrupt the performance of learned activities, such as driving, cycling, or using machines. After using this medicine, the patient should not drive, cycle, or use machines until they have fully recovered.
Consult a doctor if further information is needed.
Epistatus contains maltitol
If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking this medicine.
Epistatus contains ethanol (alcohol)
Epistatus 2.5 mg, solution for oral use
This medicine contains 49 mg of alcohol (ethanol) per dose. The amount of alcohol in the dose of this medicine is equivalent to less than 1 mL of beer or 1 mL of wine.
Epistatus 5 mg, solution for oral use
This medicine contains 99 mg of alcohol (ethanol) per dose. The amount of alcohol in the dose of this medicine is equivalent to less than 3 mL of beer or 1 mL of wine.
Epistatus 7.5 mg, solution for oral use
This medicine contains 148 mg of alcohol (ethanol) per dose. The amount of alcohol in the dose of this medicine is equivalent to less than 4 mL of beer or 2 mL of wine.
Epistatus 10 mg, solution for oral use
This medicine contains 197 mg of alcohol (ethanol) per dose. The amount of alcohol in the dose of this medicine is equivalent to less than 5 mL of beer or 2 mL of wine.
A small amount of alcohol in this medicine will not cause noticeable effects.
Epistatus contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which means the medicine is considered "sodium-free".
3. How to use Epistatus
This medicine should always be used exactly as prescribed by a doctor. In case of doubts, consult a doctor or pharmacist. The doctor treating the patient should provide the parents or caregivers with instructions on how to administer Epistatus and what to do if the seizure does not stop.
Epistatus is intended for use on the mucous membrane of the mouth, which means it can only be used in the mouth.
When administering the medicine, be careful not to choke the patient.
Depending on the child's age, they will receive the following doses from the packaging labeled with a specific color:
| Age | Dose | Color of packaging |
| From 3 to 6 months in a hospital setting | 2.5 mg (0.25 mL) | yellow |
| from 6 months to less than 1 year | 2.5 mg (0.25 mL) | yellow |
| from 1 year to less than 5 years | 5 mg (0.5 mL) | blue |
| from 5 years to less than 10 years | 7.5 mg (0.75 mL) | purple |
| from 10 years to less than 18 years | 10 mg (1 mL) | orange |
The dose is the full contents of one oral syringe. Do not administer more than one dose without consulting a doctor.
In the case of infants from 3 to 6 months of age, treatment should only be carried out in a hospital setting where monitoring and access to resuscitation equipment are possible.
After administering Epistatus, patients should remain under the supervision of a caregiver who should stay with the patient.
Do not inject Epistatus intravenously or intramuscularly. Do not attach a needle to the syringe.
Preparing the medicine for administration
If the patient has a seizure, allow their body to move freely and do not try to restrain the patient's movements. Only move the patient if they are in danger due to surrounding objects or obstacles, for example, if the patient is near a road, an open water tank, hot kitchen appliances, fire, or sharp objects.
Secure the patient's head with something soft, such as a pillow or the caregiver's knee.
How to administer the medicine
Ask a doctor, pharmacist, or nurse to show you how to take or administer this medicine. In case of any doubts, contact a doctor, pharmacist, or nurse.
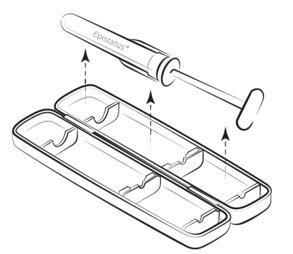
Step 1
Remove the plastic tamper-evident device from the side, open the packaging, and remove the syringe.
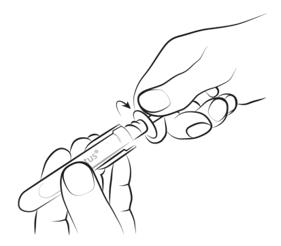
Step 2
Holding the syringe by the transparent handles, unscrew the amber cap in the opposite direction to the arrow and remove it.
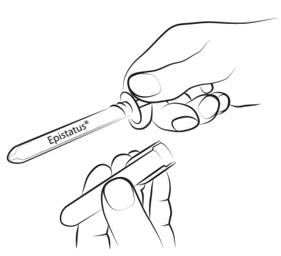
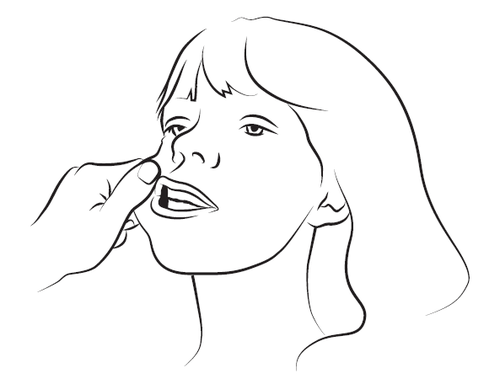
Step 3
Gently squeeze and pull the patient's cheek with your index finger and thumb. Place the tip of the syringe in the back of the space between the inner surface of the cheek and the lower gum (buccal cavity).
Step 4
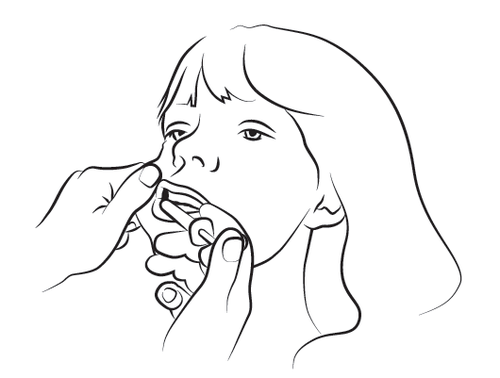
Slowly administer about half of the solution volume into the buccal cavity on one side of the mouth, and then slowly administer the rest on the other side, pressing the syringe plunger until it stops. If it is particularly difficult to introduce the syringe into the mouth on one side, administer the entire dose on one side of the mouth within 4-5 seconds. Safely dispose of the syringe and cap.
What to do if the patient's condition does not improve
Call emergency medical services immediately - call an ambulance - if the patient's seizures do not stop shortly after administering Epistatus.
Follow the doctor's instructions.
Do not administer a second dose of Epistatus without the doctor's explicit recommendation.
What to do if the patient's condition improves but the seizure recurs
Call emergency medical services immediately - call an ambulance.
Do not administer a second dose of Epistatus without the doctor's explicit recommendation.
Provide the empty syringe of the administered medicine to the ambulance crew or doctor so they know what medicine and dose were administered.
Using a higher dose of Epistatus than recommended
Call emergency medical services immediately - call an ambulance.
Symptoms that may occur after using too high a dose of Epistatus:
- Sleepiness, fatigue, feeling of exhaustion,
- Confusion or feeling of disorientation,
- Loss of coordination,
- Muscle weakness,
- Low blood pressure - may cause dizziness and fainting,
- Breathing difficulties.
Keep the syringe of the administered medicine to show it to the ambulance crew or doctor.
In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Epistatus can cause side effects, although not everybody gets them.
Serious side effects
Seek medical advice immediately or call an ambulance if any of the following side effects occur:
- Significantly worsened breathing difficulties, e.g., slow or shallow breathing or blue discoloration of the lips (cyanosis). In very rare cases, breathing may stop completely.
- Cardiac arrest (the heart stops beating) occurs very rarely. Symptoms include loss of consciousness associated with the absence of a pulse.
- Swelling of the face, lips, tongue, or throat, which makes swallowing or breathing difficult, or paleness of the skin, weak and rapid pulse, or feeling of loss of consciousness. This may indicate a severe allergic reaction.
Other side effects
Common side effects (may affect up to 1 in 10 people):
- Sleepiness or loss of consciousness, muscle spasms, and tremors (involuntary muscle twitching), reduced alertness, headache, dizziness;
- Nausea and vomiting;
- Fatigue.
Uncommon side effects (may affect up to 1 in 100 people):
- Agitation, hallucinations (seeing and sometimes hearing things that are not there);
- Transient memory loss;
- Rash, urticaria (uneven rash), itching.
Rare side effects (may affect up to 1 in 10,000 people):
- Aggression, difficulty in coordinating muscle movements, physical assault;
- Seizure (convulsions), restlessness;
- Low blood pressure, slow heart rate, or flushing of the face and neck (flushing);
- Shortness of breath;
- Constipation;
- Dry mouth;
- Hiccup.
Side effects with unknown frequency (frequency cannot be estimated from available data):
- Irritability, confusion, hostility, euphoria (excessive feeling of happiness or excitement);
- Thrombosis (blood clotting or clot formation in certain parts of the circulatory system), laryngospasm (constriction of the vocal cords causing difficulty breathing and loud breathing).
Reporting side effects
If side effects occur, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel: +48 22 492 13 01, fax: +48 22 492 13 09, website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Epistatus
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after: EXP. The expiry date refers to the last day of the month stated.
Do not store above 25°C.
Do not store in the refrigerator or freeze.
Store in the original packaging to protect from light.
Do not use this medicine if the syringe is damaged or the solution is not clear (e.g., turbidity or white particles are visible).
Medicines should not be disposed of via household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Epistatus contains
- The active substance of Epistatus is midazolam (in the form of midazolam maleate). Each 0.25 mL oral syringe contains 2.5 mg of midazolam. Each 0.5 mL oral syringe contains 5 mg of midazolam. Each 0.75 mL oral syringe contains 7.5 mg of midazolam. Each 1 mL oral syringe contains 10 mg of midazolam.
- Other ingredients are: anhydrous ethanol, sodium saccharin, glycerol, purified water, sodium hydroxide (to adjust pH), and liquid maltitol.
What Epistatus looks like and contents of the packaging
Epistatus is a clear, colorless to light yellow solution. Epistatus is available in a 1 mL transparent, colorless, plastic, single-use (needle-free) oral syringe filled (without the need for a needle) with a transparent, amber, plastic cap with different fill volumes. Each syringe contains a single dose of 0.25 mL, 0.5 mL, 0.75 mL, or 1 mL of the medicine.
Each oral syringe is supplied in an individual packaging with a seal in a polypropylene container.
Epistatus 2.5 mg, solution for oral use: yellow label on the syringe, yellow container.
Epistatus 5 mg, solution for oral use: blue label on the syringe, blue container.
Epistatus 7.5 mg, solution for oral use: purple label on the syringe, purple container.
Epistatus 10 mg, solution for oral use: orange label on the syringe, orange container.
Marketing authorization holder and manufacturer
Marketing authorization holder
SERB SA
Avenue Louise 480
1050 Brussels
Belgium
Tel. +48 22 307 03 61
Manufacturer/Importer
MoNo chem-pharm Produkte GmbH, Leystraße 129, 1-1200 Vienna, Austria
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Denmark
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
Mundhulevæske, opløsning
Finland
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
Liuos suuonteloon
Sweden
Epistatus
Slovenia
Epistatus 2.5 mg, 5 mg, 7.5 mg, 10 mg
oralna raztopina
Norway
Epistatus
Germany
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
Lösung zur Anwendung in der Mundhöhle
Greece
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
Στοματικό διάλυμα
Ireland
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
Oromucosal Solution
Italy
Epistatus
Poland
Epistatus
Hungary
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
szájnyálkahártyán alkalmazott oldat
Portugal
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
solução bucal
Austria
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
Lösung zur Anwendung in der Mundhöhle
United Kingdom (Northern Ireland)
Epistatus 2.5 mg/5 mg/7.5 mg/10 mg
oromucosal solution
Date of last revision of the leaflet: 07/2023
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterMoNo chem-pharm. Produkte GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EpistatusDosage form: Tablets, 15 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Tablets, 7.5 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Solution, 10 mgActive substance: midazolamManufacturer: MoNo chem-pharm. Produkte GmbHPrescription required
Alternatives to Epistatus in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Epistatus in Spain
Alternative to Epistatus in Ukraine
Online doctors for Epistatus
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Epistatus – subject to medical assessment and local rules.









