
Ducressa
Ask a doctor about a prescription for Ducressa

How to use Ducressa
Leaflet accompanying the packaging: information for the user
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Ducressa 1 mg/ml + 5 mg/ml
Eye drops, solution
Dexamethasone + Levofloxacin
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Ducressa and what is it used for
- 2. Important information before using Ducressa
- 3. How to use Ducressa
- 4. Possible side effects
- 5. How to store Ducressa
- 6. Contents of the packaging and other information
1. What is Ducressa and what is it used for
What kind of medicine is this and how does it work
Ducressa eye drops, solution contain levofloxacin and dexamethasone.
Levofloxacin is an antibiotic belonging to a group called fluoroquinolones (sometimes shortened to quinolones). Its action involves killing certain types of bacteria that can cause infection.
Dexamethasone is a corticosteroid with anti-inflammatory effects (it stops symptoms such as pain, feeling of heat, swelling, and redness).
What is this medicine used for?
Ducressa is used to prevent and treat inflammatory conditions and to prevent possible infection of the eye after cataract surgery in adults.
2. Important information before using Ducressa
When not to use Ducressa
- If the patient is allergic to levofloxacin (or other quinolones) or dexamethasone (or other corticosteroids), or any of the other ingredients of this medicine (listed in section 6).
- If the patient has an eye infection for which they are not taking medication, such as a viral infection (e.g., herpes simplex keratitis or chickenpox), fungal infections, or eye tuberculosis. The infection may be indicated by a sticky discharge from the eye or redness of the eye that has not been examined by a doctor.
Warnings and precautions
Before using Ducressa, you should discuss this with your doctor:
- If the patient is taking any other antibiotics, including oral antibiotics. Like other anti-infective drugs, prolonged use may lead to antibiotic resistance and, as a result, excessive growth of pathogenic microorganisms.
- If the patient has high eye pressure or has had high eye pressure after using a steroid eye medicine. Taking Ducressa may cause this phenomenon to recur. The doctor should be informed about high eye pressure.
- If the patient has glaucoma.
- If the patient has vision disturbances or blurred vision.
- If the patient is using eye non-steroidal anti-inflammatory drugs (NSAIDs), see "Ducressa and other medicines".
- If the patient has a disease that causes thinning of the eye tissues, as prolonged steroid treatment may result in further thinning and possible perforation.
- If the patient has diabetes.
Important information for patients wearing contact lenses
After cataract surgery, contact lenses should not be worn during the entire treatment period with Ducressa.
Children and adolescents
Ducressa is not recommended for children and adolescents under 18 years of age due to the lack of data on safety and efficacy in this age group.
Ducressa and other medicines
You should tell your doctor or pharmacist
- About all medicines the patient is currently taking or has recently taken, as well as medicines the patient plans to take, including those obtained without a prescription.
- About using other types of eye drops or eye ointments before starting Ducressa (see section 3 - How to use Ducressa).
- About using ophthalmic NSAIDs (for pain and inflammation of the eye), such as ketorolac, diclofenac, bromfenac, and nepafenac. Concurrent use of oral steroids and ophthalmic NSAIDs may increase the risk of eye healing problems.
- About using ritonavir or cobicistat (used to treat HIV), as they may increase the amount of dexamethasone in the blood.
- About using probenecid (for gout), cimetidine (for stomach ulcers), and cyclosporine (to prevent transplant rejection), as they may alter the absorption and metabolism of levofloxacin.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. Ducressa should not be used during pregnancy and breastfeeding.
Driving and using machines
If after a short time of using the medicine, there is temporary blurred vision, the patient should not drive vehicles or operate machines until their vision is clear.
Ducressa contains phosphate buffer
The medicine contains 4.01 mg of phosphates per ml, which corresponds to 0.12 mg per drop. In the case of severe damage to the clear layer in front of the eye (cornea), phosphate may, in very rare cases, cause the appearance of cloudy spots on the cornea, resulting from calcium accumulation during treatment. In this case, the patient should consult a doctor, who may recommend treatment with phosphate-free preparations.
Ducressa contains benzalkonium chloride
The medicine contains 0.05 mg of benzalkonium chloride per ml, which corresponds to 0.0015 mg per drop.
Benzalkonium chloride may also cause eye irritation, especially in the case of dry eyes or corneal diseases (the clear layer in front of the eye). If the patient experiences unusual sensations in the eye, stinging, or eye pain after taking this medicine, they should consult a doctor.
3. How to use Ducressa
This medicine should always be used exactly as advised by your doctor or pharmacist. If you are unsure, you should consult your doctor or pharmacist.
The recommended dose is 1 drop into the affected eye every 6 hours. The maximum dose is 4 drops per day.
The total treatment period with Ducressa usually lasts 7 days, and then, if the doctor deems it necessary, steroid eye drops should be used for the next 7 days.
The doctor will inform you how long to use the drops.
If the patient is using any other eye medicine, they should wait at least 15 minutes between using different types of drops. Eye ointments should be used last.
Instructions for use:
If possible, the patient should ask someone else to administer these drops. That other person should be asked to read this instruction together with the patient.
- 1) Wash your hands thoroughly (illustration 1).
- 2) Open the bottle. When opening the bottle for the first time, remove the loose ring from the cap. Be careful not to let the tip of the bottle with the dropper touch the eye, the skin around the eye, or fingers.
- 3) Unscrew the bottle cap. Hold the bottle upside down between your thumb and fingers.
- 4) Pull the lower eyelid down with your finger to create a "pocket" between the eyelid and the eye. The preparation should be instilled into this pocket (illustration 2).
- 5) Tilt your head back and bring the tip of the bottle close to the eye, then gently squeeze the bottle in the middle and let the drop fall into the eye (illustration 3). Remember that there may be a few-second delay between squeezing the bottle and the drop coming out. Do not squeeze the bottle too hard.
- 6) After administering Ducressa, press the corner of the eye near the nose with your finger. This helps prevent the medicine from entering the rest of the body (illustration 4).
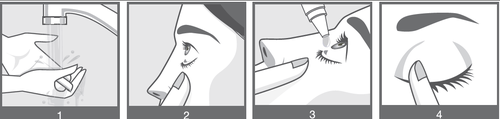
If the drop does not get into the eye, try again. Immediately after use, the bottle should be tightly closed again.
Using more than the recommended dose of Ducressa
In case of using more of this medicine than recommended, it can be rinsed with warm water.
Missing a dose of Ducressa
If a dose of the medicine is missed, do not worry, just use it as soon as possible. Do not use a double dose to make up for the missed dose.
Stopping the use of Ducressa
About stopping the use of the medicine earlier than recommended, the doctor should be informed. In case of any further doubts related to the use of this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are not serious and only affect the eyes.
- In very rare cases, this medicine may cause severe allergic reactions (anaphylactic reactions), which are accompanied by swelling and pressure in the throat and difficulty breathing.
- In case of any of these symptoms, the use of Ducressa should be stopped immediately, and a doctor should be consulted.
- In people taking fluoroquinolones orally or intravenously, especially the elderly and those treated with corticosteroids at the same time, swelling and tendon ruptures have occurred. In case of pain or swelling of the tendons (tendinitis), the use of Ducressa should be stopped.
In the eye (eyes) of the patient, some or all of the following side effects may also occur:
Very common(may affect more than 1 in 10 people):
- high eye pressure.
Common(may affect up to 1 in 10 people):
- discomfort, stinging, or irritation, burning, itching in the eye;
- blurred or impaired vision;
- mucus in the eye. Uncommon(may affect up to 1 in 100 people):
- prolonged corneal healing;
- eye infections;
- unusual sensations in the eye;
- increased tearing;
- dryness and fatigue of the eye;
- eye pain;
- distorted vision;
- swelling or redness (conjunctival hyperemia) of the front membrane of the eye (conjunctiva);
- swelling or redness of the eyelid;
- sensitivity to light;
- sticky eyelids.
Rare(may affect up to 1 in 1,000 people):
- increased pupil size;
- drooping eyelids;
- calcification of the cornea;
- tearing and feeling of sand in the eye (crystalline keratopathy);
- change in corneal thickness;
- ulcer on the cornea;
- small holes in the cornea (corneal perforation);
- swelling of the cornea (corneal edema);
- inflammation of the eye, which causes pain and redness (uveitis).
In other parts of the body, the following side effects may occur:
Uncommon(may affect up to 1 in 100 people):
- headache;
- change in taste;
- itching;
- stuffy or runny nose.
Rare(may affect up to 1 in 1,000 people):
- allergic reactions, such as skin rash. Very rare(may affect up to 1 in 10,000 people):
- facial swelling.
Frequency not known
- reduced adrenal function, which may manifest as low blood sugar, dehydration, weight loss, and disorientation;
- hormonal problems: excessive hair growth on the body (especially in women), weakness and muscle wasting, purple stretch marks on the skin, high blood pressure, irregular or absent menstruation, changes in protein and calcium levels in the body, growth retardation in children and adolescents, and swelling and weight gain of the face (known as "Cushing's syndrome").
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, the doctor, pharmacist, or nurse should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
phone: +48 22 49 21 301
fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Ducressa
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
Do not use this medicine if the plastic ring around the cap and neck is missing or broken before opening a new bottle.
Keep the bottle tightly closed. To prevent infections, the bottle should be discarded 28 days after it is first opened, and a new one should be used.
There are no special precautions for storage.
Medicines should not be disposed of via wastewater or household waste containers. You should ask your pharmacist how to dispose of medicines that are no longer used. This will help protect the environment.
6. Contents of the packaging and other information
What Ducressa contains
- The active substances of the medicine are levofloxacin in the form of levofloxacin hemihydrate and dexamethasone in the form of dexamethasone sodium phosphate. One milliliter of the solution contains 5 mg of levofloxacin and 1 mg of dexamethasone.
- The other ingredients are sodium dihydrogen phosphate monohydrate, disodium phosphate dodecahydrate, sodium citrate, benzalkonium chloride, solution, sodium hydroxide/hydrochloric acid, diluted (to adjust pH), water for injections.
What Ducressa looks like and what the pack contains
Ducressa is a clear, greenish-yellow solution that is practically free of particles, even if the dropped drops appear clear and colorless. It is supplied in a packaging containing one 5 ml bottle with an LDPE dropper and an HDPE cap in a cardboard box.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Romania, the country of export:
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer:
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Romanian export authorization number: 13368/2020/01
Parallel import authorization number: 130/25
This medicine is authorized for use in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Ducressa: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, Greece, Spain, Ireland, Iceland, Liechtenstein, Lithuania, Latvia, Netherlands, Germany, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden, Hungary, Italy, United Kingdom (Northern Ireland)
Dugressa: France
Date of leaflet approval: 07.04.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Santen Oy
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DucressaDosage form: Drops, (3 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Rafarm S.A.Prescription requiredDosage form: Drops, (5 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription requiredDosage form: Ointment, (5 mg + 0.3 mg)/gActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription required
Alternatives to Ducressa in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ducressa in Ukraine
Alternative to Ducressa in Spain
Online doctors for Ducressa
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ducressa – subject to medical assessment and local rules.














