
Dozholamidum + Timololum Stulln
Ask a doctor about a prescription for Dozholamidum + Timololum Stulln

How to use Dozholamidum + Timololum Stulln
PATIENT INFORMATION LEAFLET
Leaflet attached to the packaging: information for the user
Dorzolamidum + Timololum Stulln, 20 mg/mL + 5 mg/mL, eye drops, solution
in a single-dose container
Dorzolamidum + Timololum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Dorzolamidum + Timololum Stulln and what is it used for
- 2. Important information before using Dorzolamidum + Timololum Stulln
- 3. How to use Dorzolamidum + Timololum Stulln
- 4. Possible side effects
- 5. How to store Dorzolamidum + Timololum Stulln
- 6. Contents of the packaging and other information
1. What is Dorzolamidum + Timololum Stulln and what is it used for
Dorzolamidum + Timololum Stulln contains two active substances: dorzolamide and timolol.
- Dorzolamide belongs to a group of medicines called "carbonic anhydrase inhibitors".
- Timolol belongs to a group of medicines called "beta-adrenergic receptor blockers" (beta-adrenolytics). These medicines lower intraocular pressure through two different mechanisms.
Dorzolamidum + Timololum Stulln is recommended for the treatment of glaucoma to lower elevated intraocular pressure, when the use of eye drops containing only a beta-adrenergic receptor blocker is not sufficient.
2. Important information before using Dorzolamidum + Timololum Stulln
When not to use Dorzolamidum + Timololum Stulln:
- if the patient is allergic to dorzolamide hydrochloride, timolol maleate, or any of the other ingredients of this medicine (listed in section 6);
- if the patient currently or in the past has had respiratory disorders, such as asthma or severe chronic obstructive pulmonary disease (a serious lung disease that can cause wheezing, breathing difficulties, and/or a persistent cough);
- if the patient has a slow heart rate, heart failure, or irregular heart rhythm;
- if the patient has severe kidney disease or severe kidney function disorders or a history of kidney stones;
- if the patient has excessive acidification of the blood due to the accumulation of chloride ions in the body (hyperchloremic acidosis).
In case of doubts about whether to use this medicine, consult a doctor or pharmacist.
Warnings and precautions
Before starting to use Dorzolamidum + Timololum Stulln, discuss it with your doctor.
Tell your doctor about all current or past eye disorders and the following diseases:
- ischemic heart disease (symptoms include chest pain or a feeling of pressure in the chest, shortness of breath, or a feeling of suffocation), heart failure, low blood pressure;
- heart rhythm disorders, such as slow heart rate;
- breathing difficulties, asthma, or chronic obstructive pulmonary disease;
- diseases related to poor blood circulation (such as Raynaud's disease or Raynaud's syndrome);
- diabetes, as timolol may mask the subjective and objective symptoms of hypoglycemia (low blood sugar);
- hyperthyroidism, as timolol may mask its subjective and objective symptoms.
Before surgery, inform the anesthesiologist about the use of Dorzolamidum + Timololum Stulln, as timolol may affect the action of some anesthetics.
Also, tell your doctor about any allergies or allergic reactions, including hives, facial swelling, lip swelling, tongue and/or throat swelling, which can cause difficulty breathing or swallowing.
Tell your doctor if you have experienced muscle weakness or been diagnosed with myasthenia gravis (myasthenia gravis).
If you experience eye irritation or any new eye problems, such as eye redness or eyelid swelling, seek medical attention immediately.
If you suspect that Dorzolamidum + Timololum Stulln is causing an allergic reaction or hypersensitivity (e.g., skin rash, severe skin reaction, or skin redness and eye itching), discontinue use of this medicine and contact your doctor immediately.
Tell your doctor if you have an eye infection, have had eye trauma, have had eye surgery, or experience a reaction accompanied by the appearance of new or worsening symptoms.
After administration to the eye, Dorzolamidum + Timololum Stulln may cause systemic effects.
No studies have been conducted on the use of Dorzolamidum + Timololum Stulln in patients using contact lenses.
Before using this medicine, people who use soft contact lenses should consult their doctor.
Children
Experience with the use of eye drops containing dorzolamide and timolol with a preservative in infants and children is limited.
Use in the elderly
Studies conducted with eye drops containing dorzolamide and timolol with a preservative have shown similar effects in the elderly and younger patients.
Use in patients with liver function disorders
Tell your doctor about any current or past liver diseases.
Dorzolamidum + Timololum Stulln and other medicines
Dorzolamidum + Timololum Stulln may affect the action of other medicines or other medicines used by the patient may affect the action of Dorzolamidum + Timololum Stulln. This also applies to other anti-glaucoma eye medicines. Tell your doctor about the use of or planned use of medicines that lower blood pressure, heart medicines, or anti-diabetic medicines. Tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take. This is especially important in the case of:
- taking medicines that lower blood pressure or are used for heart diseases (such as calcium channel blockers, beta-adrenergic receptor blockers, or digoxin);
- taking medicines used for heart rhythm disorders or restoring a regular heart rhythm (such as calcium channel blockers, beta-adrenergic receptor blockers, or digoxin);
- using other eye drops containing beta-adrenergic receptor blockers;
- taking other carbonic anhydrase inhibitors, such as acetazolamide;
- taking monoamine oxidase inhibitors (MAOIs);
- taking medicines with a parasympathomimetic (cholinergic) effect, which may be used for urinary disorders. Parasympathomimetic medicines are also sometimes used to restore normal bowel motility;
- taking narcotics, such as morphine, used to treat moderate to severe pain;
- taking anti-diabetic medicines;
- taking antidepressants, such as fluoxetine and paroxetine;
- taking chemotherapeutic agents from the sulfonamide group;
- taking quinidine (a medicine used to treat heart diseases and certain types of malaria).
Pregnancy and breastfeeding
Before using any medicine, consult a doctor or pharmacist.
Use during pregnancy
Do not use Dorzolamidum + Timololum Stulln during pregnancy, unless your doctor considers it necessary.
Use during breastfeeding
Do not use Dorzolamidum + Timololum Stulln during breastfeeding. Timolol may pass into breast milk.
Before taking any medicine during breastfeeding, consult your doctor.
Driving and using machines
No studies have been conducted on the effect of the medicine on the ability to drive or use machines. Some side effects of Dorzolamidum + Timololum Stulln, such as blurred vision, may affect the ability to drive or use machines. Patients who feel unwell or have blurred vision should not drive or use machines.
3. How to use Dorzolamidum + Timololum Stulln
This medicine should always be used as directed by your doctor. If you have any doubts, consult your doctor or pharmacist. Your doctor will determine the correct dose and duration of treatment.
The recommended dose is one drop into the affected eye(s) in the morning and evening.
If you are using other eye drops in addition to Dorzolamidum + Timololum Stulln, wait at least 10 minutes before administering the next medicine.
Do not change the dose of the medicine without consulting your doctor.
If you have difficulty applying the drops, ask a family member or caregiver for help.
Do not let any part of the single-dose container touch the eye or its surroundings. This can lead to eye injury and contamination of the container with bacteria, which can cause eye infection leading to serious damage or even vision loss. To avoid possible contamination of the single-dose container, wash your hands before using the medicine and avoid touching the tip of the single-dose container to any surface. A new single-dose container should be opened just before each use. Each single-dose container contains enough solution for one dose of the medicine for both eyes, if your doctor has prescribed it for both eyes.
Discard the opened single-dose container immediately after use.
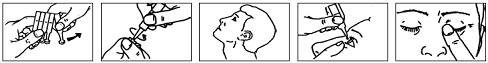
Instructions for use
- 1. Wash your hands.
- 2. Open the aluminum sachet and remove the blister pack of single-dose containers.
- 3. Tear off one single-dose container from the blister pack (Fig. 1).
- 4. Open the single-dose container by twisting off the tip. Do not touch the tip after opening the single-dose container (Fig. 2).
- 5. Tilt your head back (Fig. 3).
- 6. Pull down the lower eyelid with your finger and hold the single-dose container in your other hand. Squeeze the single-dose container so that one drop falls into the eye (Fig. 4).
- 7. Close your eye and press the inner corner of your eye with your finger for about 2 minutes. This can prevent the medicine from entering the entire body (Fig. 5). If necessary, repeat the steps from 5 to 7 to administer the medicine to the other eye.
- 8. Discard the single-dose container after use.
- 9. Put the remaining single-dose containers back into the sachet and close it by folding the edge. Place the sachet in the carton. If there are still single-dose containers in the sachet after 3 months from the opening date, discard them in an environmentally safe manner and open a new sachet. It is essential to use the eye drops continuously as directed by your doctor.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Using a higher dose of Dorzolamidum + Timololum Stulln than recommended
In case of administration of too many drops or ingestion of the contents of the single-dose container, among other symptoms, dizziness, breathing difficulties, or a feeling of slow heart rate may occur. Contact your doctor immediately.
Missing a dose of Dorzolamidum + Timololum Stulln
Dorzolamidum + Timololum Stulln should be used as directed by your doctor.
If you miss a dose, use it as soon as possible. However, if it is almost time for your next dose, do not use the missed dose and return to your regular dosing schedule.
Do not use a double dose to make up for a missed dose.
Stopping the use of Dorzolamidum + Timololum Stulln
Before stopping the medicine, consult your doctor.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Treatment with eye drops can usually be continued, unless the side effects are severe. If you are concerned, contact your doctor or pharmacist. Do not stop using Dorzolamidum + Timololum Stulln without consulting your doctor.
Generalized allergic reactions, including swelling under the skin, can occur in the face and limbs and cause respiratory tract obstruction and difficulty swallowing or breathing, hives, itching rash, local and generalized rash, itching, and severe, life-threatening allergic reactions.
During clinical trials or after the marketing of the medicinal product, the following side effects have been reported in relation to eye drops containing dorzolamide and timolol without preservatives or with one of their active substances:
Very common (may affect more than 1 in 10 people):
Burning and stinging sensation in the eye, change in taste sensation
Common (may affect up to 1 in 10 people):
Redness of the eyeball and skin around the eye, tearing or itching of the eye, corneal erosion (damage to the front layer of the eyeball), swelling and/or irritation of the eyeball and skin around the eye, foreign body sensation in the eye, decreased sensitivity of the cornea (not feeling a foreign body in the eye and not feeling pain), eye pain, dry eyes, blurred vision, headache, sinusitis (feeling of tension or fullness in the nose), nausea, weakness, and fatigue.
Uncommon (may affect up to 1 in 100 people):
Dizziness, depression, uveitis, vision disorders, including changes in refraction (in some cases due to the withdrawal of miotic medicines), slow heart rate, fainting, breathing difficulties (shortness of breath), indigestion, and kidney stones (often characterized by sudden onset in the form of severe, crampy pain in the lower back and/or side, groin, or abdomen).
Rare (may affect up to 1 in 1000 people):
Systemic lupus erythematosus (an autoimmune disease that can cause inflammation of internal organs), tingling or numbness of the hands or feet, insomnia, nightmares, memory loss, worsening of myasthenia symptoms (muscle disorder), decreased libido, stroke, transient myopia, which may resolve after discontinuation of the medicine, detachment of the layer under the retina, which can cause vision disorders, drooping eyelids (eyelids are half-closed), double vision, formation of crusts on the eyelids, corneal edema (with subjective symptoms of vision disorders), low eye pressure, ringing in the ears, low blood pressure, irregular heart rhythm, changes in heart rhythm or rate, congestive heart failure (heart disease characterized by shortness of breath and swelling of the feet and legs due to fluid accumulation), edema (fluid accumulation), cerebral ischemia (reduced blood flow to the brain), chest pain, rapid or irregular heartbeat (palpitations), myocardial infarction, Raynaud's disease, swelling of the hands and feet or cold hands and feet, and impaired circulation in the upper and lower limbs, muscle cramps in the legs and/or leg pain when walking (claudication), shortness of breath, respiratory failure, rhinitis (nasal discharge or feeling of a stuffy nose), nosebleeds, bronchospasm, cough, throat irritation, dry mouth, contact dermatitis, hair loss, white-silvery rash (psoriasis-like rash), Peyronie's disease (which can cause penile curvature), allergic reactions, such as rash, hives, itching, and in rare cases, swelling of the lips, eyelids, and mouth, wheezing, or severe skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis).
Like other locally administered eye medicines, timolol is absorbed into the bloodstream, which can cause side effects similar to those observed after oral administration of beta-adrenergic receptor blockers. Side effects occur less frequently after local administration of eye drops than after oral administration or injections of these medicines.
Among the additional side effects listed, reactions typical of the therapeutic group of beta-adrenolytic medicines used in ophthalmic disorders are included.
Frequency not known (cannot be estimated from the available data)
Low blood sugar levels, hallucinations, heart failure, type of heart rhythm disorder, rapid heart rate, increased blood pressure, abdominal pain, vomiting, muscle pain unrelated to physical exertion, sexual function disorders.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301, Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Dorzolamidum + Timololum Stulln
Keep the medicine out of the sight and reach of children.
Do not store above 30°C.
Store in the original packaging to protect from light.
Do not use the medicine for more than 3 months after opening the aluminum sachet.
Dorzolamidum + Timololum Stulln does not contain a preservative. After opening the single-dose container, the contents should be used immediately and not stored. Discard the solution remaining in the single-dose container after use.
Do not use this medicine after the expiry date stated on the carton, sachet, and single-dose container after: Expiry date. The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Dorzolamidum + Timololum Stulln contains
- The active substances of the medicine are dorzolamide and timolol. Each milliliter of solution contains 20 mg of dorzolamide (22.26 mg of dorzolamide hydrochloride) and 5 mg of timolol (6.83 mg of timolol maleate).
- The other ingredients are: hydroxyethylcellulose (4000 - 5000 mPa∙s), mannitol, sodium citrate, sodium hydroxide (to adjust pH), and water for injections.
What Dorzolamidum + Timololum Stulln looks like and contents of the packaging
Dorzolamidum + Timololum Stulln is a clear, almost colorless, slightly viscous solution, practically free from visible particles. Each single-dose container contains 0.2 mL or 0.3 mL of solution.
Dorzolamidum + Timololum Stulln is available in packs containing 10, 20, 30, 50, 60, 100, or 120 single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Pharma Stulln GmbH
Werksstrasse 3
92551 Stulln
Germany
Pharm Supply Sp. z o.o.
Marconich Street 2/9
02-954 Warsaw
Phone: (+48) 22 6423331
Email: [email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Dorzolamid + Timolol Stulln sine
Austria
DORTIRUS
France
Dorzocomp-Stulln sine
Germany
DORZYLEA
Greece
Dorzolamide + Timolol Stulln zonder conserveermiddel
Netherlands
Dorzolamidum + Timololum Stulln
Poland
Dorzolamida/ Timolol Stulln PF
Spain
Date of last revision of the leaflet:November 2023
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterPharma Stulln GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Dozholamidum + Timololum StullnDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Dozholamidum + Timololum Stulln in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Dozholamidum + Timololum Stulln in Hiszpania
Alternative to Dozholamidum + Timololum Stulln in Ukraina
Online doctors for Dozholamidum + Timololum Stulln
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Dozholamidum + Timololum Stulln – subject to medical assessment and local rules.














