
Diklofenak Viatris

How to use Diklofenak Viatris
Package Leaflet: Information for the User
Diklofenak Viatris, 180 mg, Medicinal Patch (1.3%)
Diclofenacum epolaminum
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient, or as directed by a doctor or pharmacist.
- Keep this leaflet, so you can read it again if you need to.
- If you need advice or additional information, ask your pharmacist.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- If after a short treatment period there is no improvement or the patient feels worse, they should contact their doctor.
Table of Contents of the Leaflet
- 1. What is Diklofenak Viatris and what is it used for
- 2. Important information before using Diklofenak Viatris
- 3. How to use Diklofenak Viatris
- 4. Possible side effects
- 5. How to store Diklofenak Viatris
- 6. Contents of the pack and other information
1. What is Diklofenak Viatris and what is it used for
Diklofenak Viatris contains the active substance diclofenac epolamine and belongs to a group of medicines used to relieve pain and inflammation, known as nonsteroidal anti-inflammatory drugs (NSAIDs).
Diklofenak Viatris is used on the skin to relieve pain and inflammation of rheumatic or traumatic origin in joints, muscles, tendons, or ligaments.
If after a short treatment period with Diklofenak Viatris there is no improvement or the patient feels worse, they should contact their doctor.
2. Important information before using Diklofenak Viatris
When not to use Diklofenak Viatris:
- if the patient is allergic to diclofenac or any of the other ingredients of this medicine (listed in section 6)
- if the patient is allergic to acetylsalicylic acid (aspirin) or other nonsteroidal anti-inflammatory drugs (NSAIDs)
- in the last three months of pregnancy (see "Pregnancy, breastfeeding, and fertility")
- if the patient has asthma, breathing problems, skin rash, or hay fever after using acetylsalicylic acid (aspirin) or other NSAIDs
- if the skin at the planned patch application site is damaged or injured, with open wounds, infected, weeping, or eczematous lesions
- if the patient has active stomach ulcers (peptic ulcers)
- in children and adolescents under 16 years of age.
Warnings and precautions
Before starting treatment with Diklofenak Viatris, the patient should discuss it with their doctor or pharmacist:
- if the patient has breathing problems (allergic rhinitis, nasal polyps, asthma, chronic bronchitis);
- if the patient has kidney, heart, or liver disease;
- if the patient has had stomach or duodenal ulcers;
- if the patient has had inflammatory bowel disease, such as Crohn's disease or ulcerative colitis;
- if the patient has an increased tendency to bleeding from the gut;
- if the patient is breastfeeding or in the first six months of pregnancy.
Other important information
The patient should always use the smallest effective dose of Diklofenak Viatris for the shortest time necessary to control symptoms.
Caution should be exercised when using Diklofenak Viatris in elderly patients, as the likelihood of side effects may be greater.
To minimize the frequency of side effects, the smallest effective dose should be used for the shortest possible time.
Diklofenak Viatris should not be used at the same time as other medicines containing diclofenac or other NSAIDs.
The patient should avoid exposure to direct sunlight or sunlamps (tanning beds) for at least one day after removing the patch, as this will reduce the risk of an adverse reaction to light (photosensitivity).
Like other locally applied medicines, Diklofenak Viatris may cause skin redness and itching (skin sensitization), especially with prolonged use. If a skin reaction occurs at the patch application site, treatment should be discontinued and the patient should consult their doctor or pharmacist.
Elderly
Greater care should be taken in elderly patients when using Diklofenak Viatris, as the likelihood of side effects may be greater.
Patients with liver or kidney problems
Patients with liver or kidney problems should be cautious when using Diklofenak Viatris, as the likelihood of side effects may be greater.
Children and adolescents
Diklofenak Viatris should not be used in children under 16 years of age.
Diklofenak Viatris and other medicines
When used correctly, the risk of interactions with other medicines is very low. However, the patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. In particular, the patient should inform their doctor if they are taking other medicines containing diclofenac or other NSAIDs.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant, breastfeeding, or thinks they may be pregnant, they should consult their doctor before using this medicine. Diklofenak Viatris should not be used if the patient is starting the sixth month of pregnancy or after the sixth month of pregnancy, as it may harm the unborn baby or cause complications during delivery.
Before the sixth month of pregnancy, Diklofenak Viatris should only be used on the advice of a doctor, in the smallest possible dose, and for the shortest possible time.
Breastfeeding
During breastfeeding, Diklofenak Viatris should only be used on the advice of a doctor, as diclofenac passes into breast milk in small amounts. The medicine should not be used during breastfeeding on the breasts of nursing mothers or on any relatively large area of skin or for a prolonged period.
Fertility
If the patient has problems getting pregnant and is using Diklofenak Viatris, they should tell their doctor, as it may be necessary to stop treatment.
Driving and using machines
Diklofenak Viatris has no effect on the ability to drive or use machines.
Diklofenak Viatris contains propylene glycol, methyl parahydroxybenzoate, and propyl parahydroxybenzoate
- This medicine contains 420 mg of propylene glycol in each medicinal patch.
- Methyl parahydroxybenzoate and propyl parahydroxybenzoate may cause allergic reactions (possibly delayed).
3. How to use Diklofenak Viatris
This medicine should always be used exactly as described in the package leaflet for the patient, or as directed by a doctor or pharmacist. If in doubt, the patient should ask their doctor or pharmacist.
Adults
The recommended dose is 1 or 2 patches per day, applied every 12 or 24 hours, for no longer than 14 days. The patches should not be cut. If after 14 days of treatment there is no improvement or the patient feels worse, they should contact their doctor.
Adolescents from 16 to 18 years of age
The recommended dose is 1 or 2 patches per day, applied every 12 or 24 hours, for no longer than 7 days. The patches should not be cut. If after 7 days of treatment there is no improvement or the patient feels worse, they should contact their doctor.
Children and adolescents under 16 years of age
The medicinal patch should not be used in children and adolescents under 16 years of age due to the lack of sufficient data on efficacy and safety.
Elderly
Caution should be exercised, as the likelihood of side effects may be greater in elderly patients.
Patients with liver or kidney problems
Information on the use of medicinal patches containing diclofenac in patients with liver or kidney problems can be found in the section "Special warnings and precautions for use".
How to use Diklofenak Viatris
Cut open the top of the sachet and remove the patch.
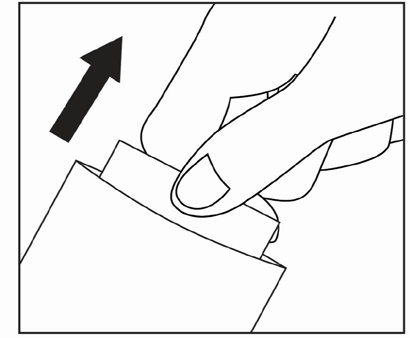
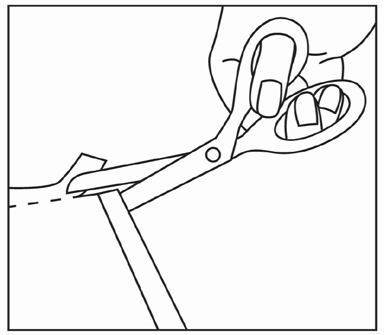
Close the sachet carefully.
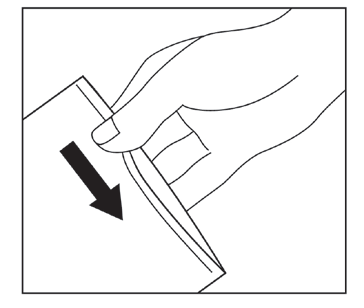
Remove the protective film from the adhesive surface.
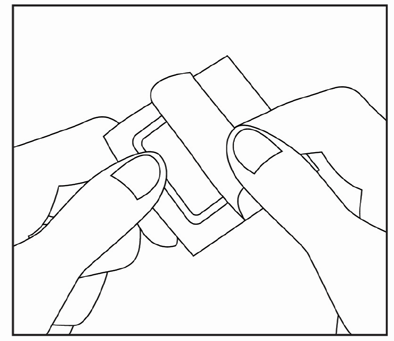
Apply the patch to the skin at the painful or swollen area.
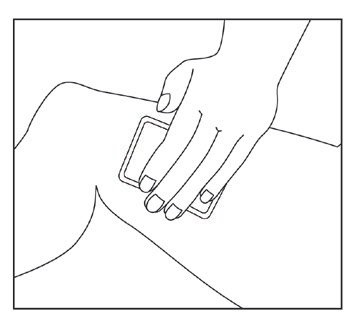
- If necessary, the patch can be secured with an elastic net or bandage. These can be purchased at a pharmacy.
- Do not cover the patch with airtight plastic or a bandage (occlusive dressing).
- The medicinal patch should only be applied to undamaged skin without lesions, avoiding scratched skin and open wounds, and should not be used during bathing or showering.
- Avoid contact of the patch with the eyes, nose, mouth, genital area, and anus. If contact occurs with these areas, they should be rinsed with water.
- The patches should not be cut.
- The patch should be changed.
- The patch should be removed every 12 or 24 hours and a new one applied.
In case of using a higher dose of Diklofenak Viatris than recommended, systemic (whole body) side effects may occur, such as stomach or gut disorders. In such cases, the patient should immediately contact their doctor or go to the nearest hospital, as additional measures may be necessary.
Missing a dose of Diklofenak Viatris
The patient should not use more than one patch at the same time. The patient should not use an additional patch to make up for a missed dose. For more information, the patient should ask their doctor or pharmacist.
4. Possible side effects
Like all medicines, Diklofenak Viatris can cause side effects, although not everybody gets them.
If the patient experiences any of the following symptoms of an allergic reaction, they should STOP USINGDiklofenak Viatris and inform their doctor or pharmacist:
Very rare(may affect up to 1 in 1000 people)
- Swelling of the lips, eyes, or tongue, wheezing, or asthma attack, which are symptoms of an acute allergic reaction
Common(may affect up to 1 in 10 people)
- Burning or stinging at the patch application site.
Side effects usually occur at the site of application.
Common(may affect up to 1 in 10 people)
Skin reactions: skin rash, skin inflammation, redness and swelling of the skin, itching (pruritus).
Other possible side effects
Rare(may affect up to 1 in 1000 people): skin rash with blistering, dry skin.
Very rare(may affect up to 1 in 10,000 people): allergic reaction also after exposure to sunlight or sunlamps (photosensitivity), hives.
Frequency not known(frequency cannot be estimated from the available data): burning sensation at the application site.
If the medicinal patches are used correctly, the risk of side effects is very low, but it cannot be ruled out that systemic side effects may occur with prolonged use or when used together with other medicines containing diclofenac, especially when taken orally.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department for the Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 4921 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Diklofenak Viatris
The medicine should be kept out of sight and reach of children.
The medicine should not be used after 4 months from the date of first opening the sachet. After removing each patch, the pouch should always be closed tightly. Store in the original packaging to protect from light and drying. There are no special instructions for storage temperature.
The medicine should not be used after the expiry date (EXP) stated on the sachet and carton. The expiry date refers to the last day of the month.
Unused medicinal patches should not be disposed of via wastewater or household waste. Before disposal, used patches should be folded in half, with the adhesive side inward.
Used patches should not be flushed down the toilet or put into the drainage system.
The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Diklofenak Viatris contains
The active substance is diclofenac epolamine. Each medicinal patch contains a total of 180 mg of diclofenac epolamine, which corresponds to 140 mg of diclofenac sodium (1.3% w/w).
The other ingredients are:
Outer protective layer: polyester fabric.
Adhesive layer: liquid sorbitol (non-crystallizing), 1,3-butylene glycol, sodium polyacrylate, heavy kaolin, sodium carmellose, propylene glycol (see section 2 "Diklofenak Viatris contains"), gelatin, povidone (K90), tartaric acid, titanium dioxide (E171), aluminum glycinate, polysorbate 80, disodium edetate (E385), methyl parahydroxybenzoate (E218) (see section 2 "Diklofenak Viatris contains"), propyl parahydroxybenzoate (E216) (see section 2 "Diklofenak Viatris contains"), purified water.
Protective layer (removed): polypropylene
What Diklofenak Viatris looks like and contents of the pack
Each medicinal patch consists of a white or yellowish paste spread evenly in a single layer on a backing film with a protective layer covering the adhesive surface. The sachets for repeated opening contain 5 patches.
Diklofenak Viatris is available in cartons containing 5 or 10 patches.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Cooper Consumer Health B.V.
Verrijn Stuartweg 60
1112AX Diemen
Netherlands
Importer
MIAT S.p.A.
Piazza Pasolini 2
20159 Milano
Italy
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Hungary
Diclofenac epolamine Mylan 180 mg gyógyszeres tapasz
Estonia
Diclofenac Mylan
Lithuania
Diclofenac Mylan 180 mg vaistinis pleistras
Slovakia
DicloMyl 180 mg liečivá náplasť
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterMIAT S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Diklofenak ViatrisDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Laboratorios Basi - Industria Farmaceutica, S.A.Prescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Sandoz GmbHPrescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacPrescription not required
Alternatives to Diklofenak Viatris in other countries
The best alternatives with the same active ingredient and therapeutic effect.




