
Diclofenac sodium Nutra Essential

How to use Diclofenac sodium Nutra Essential
Patient Information Leaflet: User Information
Diclofenac Sodium Nutra Essential, 40 mg/mL, Skin Spray, Solution
Diclofenac Sodium
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient information leaflet or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 3 days there is no improvement or you feel worse, consult a doctor.
Table of Contents of the Leaflet:
- 1. What is Diclofenac Sodium Nutra Essential and what is it used for
- 2. Important information before using Diclofenac Sodium Nutra Essential
- 3. How to use Diclofenac Sodium Nutra Essential
- 4. Possible side effects
- 5. How to store Diclofenac Sodium Nutra Essential
- 6. Contents of the pack and other information
1. What is Diclofenac Sodium Nutra Essential and what is it used for
Diclofenac Sodium Nutra Essential contains the active substance diclofenac sodium, which belongs to the group of nonsteroidal anti-inflammatory drugs (NSAIDs), and should be applied to the affected skin surface.
This medicine is used in adults and adolescents over 14 years of age for short-term local symptomatic treatment of mild or moderate painful and inflammatory conditions after acute, blunt trauma to small and medium joints and surrounding structures.
If after 3 days there is no improvement or you feel worse, consult a doctor.
2. Important information before using Diclofenac Sodium Nutra Essential
When not to use Diclofenac Sodium Nutra Essential
- If you are allergic to diclofenac or any of the other ingredients of this medicine (listed in section 6).
- If you are allergic to peanuts or soy, as the medicine contains soy lecithin.
- If you have ever had an allergic reaction with difficulty breathing (asthma), skin rash, hay fever, facial or tongue swelling in response to any medicine used to treat pain, fever, or inflammation, such as ibuprofen or acetylsalicylic acid (also used as an anti-coagulant).
- On open wounds, inflammation, or infected skin, as well as on eczema or mucous membranes.
- In the last trimester of pregnancy - see section "Pregnancy and breastfeeding".
Warnings and precautions
Before starting to use Diclofenac Sodium Nutra Essential, discuss it with your doctor or pharmacist.
- If you have been diagnosed with asthma or allergies.
- If the spray accidentally gets into your eyes, rinse them thoroughly with clean water and inform your doctor.
- Never swallow Diclofenac Sodium Nutra Essential.
- Stop using Diclofenac Sodium Nutra Essential if any skin rash appears after application.
- Do not cover the treated skin surface with bandages or occlusive dressings (waterproof or non-breathable).
- Do not expose the treated areas to the sun or use sun lamps.
Systemic side effects associated with the use of Diclofenac Sodium Nutra Essential cannot be ruled out if the medicine is used on large skin surfaces and for a long time. Side effects can be minimized by using the smallest effective dose for the shortest necessary period to control symptoms.
Hazards: flammable liquid. Store away from heat sources, hot surfaces, sparks, open flames, and other ignition sources.
Children and adolescents
Do not use this medicine in children and adolescents under 14 years of age.
Diclofenac Sodium Nutra Essential and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Before using Diclofenac Sodium Nutra Essential, discuss it with your doctor:
- If you are taking any medicines containing diclofenac, acetylsalicylic acid, or any other NSAID, such as ibuprofen.
Using other NSAIDs (including acetylsalicylic acid or ibuprofen) at the same time as Diclofenac Sodium Nutra Essential may increase the risk of side effects.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
Pregnancy
Diclofenac Sodium Nutra Essential should not be used in the last trimester of pregnancy, as it may increase the risk of complications in the mother and newborn.
Diclofenac Sodium Nutra Essential should only be used on the advice of a doctor during the first 6 months of pregnancy. In such cases, use the smallest effective dose and the shortest treatment period.
After oral administration of Diclofenac Sodium Nutra Essential (e.g., tablets), side effects may occur in the unborn child. It is not known whether the same risk applies to Diclofenac Sodium Nutra Essential when used on the skin/in the mouth.
Breastfeeding
Diclofenac Sodium Nutra Essential should not be used during breastfeeding, unless advised by a doctor.
Diclofenac Sodium Nutra Essential should not be applied to the breasts of nursing mothers or to large skin surfaces and for a long time.
Driving and using machines
Using Diclofenac Sodium Nutra Essential on the skin does not affect the ability to drive or operate machines.
Diclofenac Sodium Nutra Essential contains propylene glycol (E1520), ethanol, and soy lecithin:
The medicine contains 30 mg of propylene glycol (E 1520) in each dose after pressing the pump.
The medicine contains 6.65 mg of alcohol (ethanol) in each dose after pressing the pump.
The medicine contains soy oil. Do not use this medicine if you are hypersensitive to peanuts or soy.
3. How to use Diclofenac Sodium Nutra Essential
This medicine should always be used exactly as described in the patient information leaflet or as directed by a doctor or pharmacist. If you are unsure, consult a doctor or pharmacist.
The recommended dose is:
Adults and adolescents over 14 years of age:
Apply 4-5 pump sprays 3 times a day at regular intervals. The number of pump sprays depends on the size of the affected skin surface. The maximum single dose should not exceed 5 pump sprays. The maximum daily dose is 15 pump sprays.
Treatment can be stopped when symptoms (pain and swelling) disappear.
Do not use the medicine for more than 7 days without consulting a doctor first.
Consult a doctor if symptoms worsen or do not improve after 3 days of treatment.
Use in children and adolescents:
Do not use in children and adolescents under 14 years of age.
Method of use:
Diclofenac Sodium Nutra Essential is for skin use only.
Follow the instructions:
- Remove the transparent protective cap from the pump.
- Before first use, press the pump 4 times to activate it, which will cause the contents to be dispensed. Incomplete filling of the pump may lead to a smaller dose of the medicine being applied during the first use.
- Wash and dry the painful area. Apply the spray to the affected parts of the body and gently rub into the skin. After application, wipe your hands with a paper towel and then wash them, unless your hands are the area being treated.
- If too much spray is accidentally applied, wipe off the excess with a paper towel.
- Discard the paper towel in household waste to prevent the unused product from entering the water environment.
- Before applying a bandage, allow the spray to dry on the skin for a few minutes.
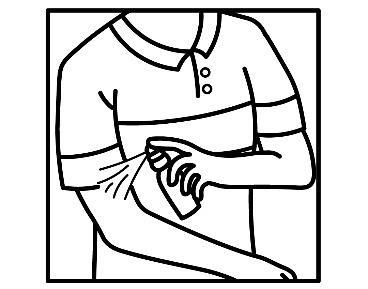
Wash and dry the painful skin surface
Apply to the skin surface to be treated
Gently massage to stimulate absorption
Using a larger dose of Diclofenac Sodium Nutra Essential than recommended
If you use more medicine than recommended, wipe off the excess with a paper towel.
If you swallow the spray, immediately inform your doctor or go to the nearest hospital. Take the bottle and leaflet with you.
Missing a dose of Diclofenac Sodium Nutra Essential
If you miss a dose of this medicine, apply it as soon as possible and continue treatment as before. Do not use a double dose to make up for the missed dose.
If you have any further questions about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Diclofenac Sodium Nutra Essential if any skin rash appears. When using topical diclofenac-containing medicines, local allergic reactions at the application site are often reported: rashes, itching, redness, burning, or dryness of the skin.
Some rare and very rare side effects may be serious:
If you experience any of the following symptoms of an allergic reaction, stop using the medicine
and seek medical advice immediately.
- Rare (may affect up to 1 in 1000 people): Skin rash with blisters.
- Very rare (may affect up to 1 in 10,000 people): Wheezing, shortness of breath, or feeling of tightness in the chest (asthma). Allergic reactions, including hives (red stripes, raised bumps often itching and burning). Swelling of the face, lips, tongue, or throat. Other side effects may occur, but they are usually mild and temporary. Inform your doctor or pharmacist immediately if you experience any of them.
- Common (may affect up to 1 in 10 patients): Skin rash, itching, redness, or burning of the skin after application of the medicine.
- Uncommon (may affect up to 1 in 100 people): Dandruff, dry skin, swelling.
- Very rare (may affect up to 1 in 10,000 people): The skin may be more sensitive to the sun (photosensitivity). Possible symptoms are sunburn with itching, swelling, and blisters. Pustular rash. Gastrointestinal disorders.
- Systemic side effects, such as pain and stomach disorders, heartburn, liver or kidney disorders, and allergic reactions, may occur if the medicine is used for a long time or on large skin surfaces.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Diclofenac Sodium Nutra Essential
Keep the medicine out of the sight and reach of children.
Store in the original packaging to protect from light.
Do not use this medicine after the expiry date stated on the carton and bottle after EXP. The expiry date refers to the last day of the month stated.
Do not use this medicine after 6 months of first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Contents of Diclofenac Sodium Nutra Essential:
- The active substance of the medicine is diclofenac sodium. Each mL of the spray solution (which corresponds to 5 pump sprays) contains 40 mg of diclofenac sodium. Each dose after pressing the pump (0.2 mL of the solution) contains 8 mg of diclofenac sodium.
- The other ingredients of the medicine are: propylene glycol (E 1520), isopropyl alcohol, phospholipon 90G (containing soybean lecithin-derived phosphatidylcholine and lysophosphatidylcholine, non-polar lipids, and tocopherol), ethanol 96%, disodium phosphate, sodium dihydrogen phosphate dihydrate, disodium edetate, ascorbyl palmitate, peppermint essential oil, hydrochloric acid diluted to adjust pH, sodium hydroxide 10% to adjust pH, and purified water.
What Diclofenac Sodium Nutra Essential looks like and contents of the pack:
Diclofenac Sodium Nutra Essential is a yellowish solution.
A 30 ml glass bottle with 25 g of spray solution, corresponding to 125 pump sprays, equipped with a polypropylene spray pump and a polyethylene dip tube.
Marketing authorization holder and manufacturer
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Medinfar Manufacturing, S.A.
Parque Industrial Armando Martins Tavares,
Rua Outeiro da Armada, 5,
3150-194 Condeixa-a-Nova
Portugal
FARMALIDER, S.A.
Calle Aragoneses 2,
28108 Alcobendas, Madrid
Spain
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterFarmalider S.A. Medinfar Manufacturing S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Diclofenac sodium Nutra EssentialDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Laboratorios Basi - Industria Farmaceutica, S.A.Prescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacManufacturer: Sandoz GmbHPrescription not requiredDosage form: Gel, 10 mg/gActive substance: diclofenacPrescription not required
Alternatives to Diclofenac sodium Nutra Essential in other countries
The best alternatives with the same active ingredient and therapeutic effect.




