
Dexamitrex

Ask a doctor about a prescription for Dexamitrex

How to use Dexamitrex
Leaflet attached to the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
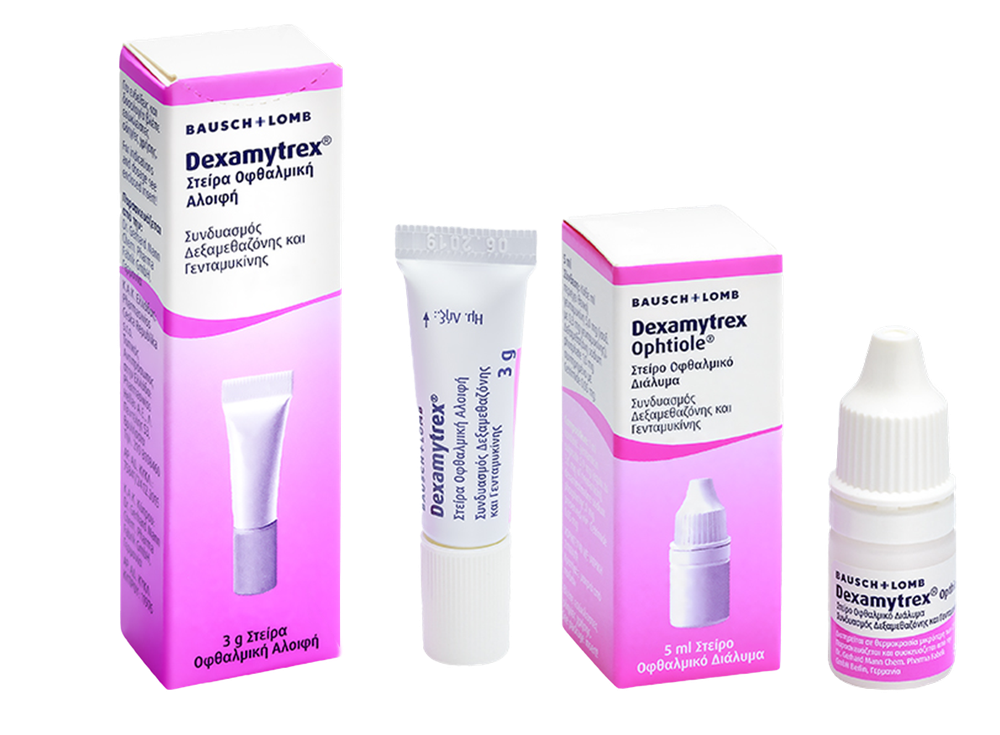
Dexamytrex(Dexamytrex Ophtiole)
(5 mg + 1 mg)/ml, eye drops, solution
Gentamicin sulfate + Dexamethasone sodium phosphate
Dexamytrex and Dexamytrex Ophtiole are different trade names for the same drug.
WARNING!The strength of 3 mg/ml regarding gentamicin on the immediate packaging is
equivalent to the 5 mg/ml notation, taking into account the content of gentamicin sulfate.
You should carefully read the contents of the leaflet before using the drug, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This drug has been prescribed to a specific person. It should not be given to others. The drug may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Dexamytrex and what is it used for
- 2. Important information before using Dexamytrex
- 3. How to use Dexamytrex
- 4. Possible side effects
- 5. How to store Dexamytrex
- 6. Contents of the packaging and other information
1. What is Dexamytrex and what is it used for
Dexamytrex contains the active substances: gentamicin sulfate - an antibiotic from the
aminoglycoside group and dexamethasone sodium phosphate - a glucocorticosteroid.
The drug is used in infections of the anterior segment of the eye, caused by microorganisms sensitive to
gentamicin, such as bacterial conjunctivitis, keratitis - without corneal damage and eyelid margin damage with severe inflammation, as well as in secondarily infected allergic conjunctivitis and eyelid margin inflammation.
2. Important information before using Dexamytrex
When not to use Dexamytrex:
- if the patient is allergic to gentamicin, dexamethasone, or any of the other ingredients of this drug (listed in section 6);
- if the patient has an acute, purulent disease of the anterior segment of the eye;
- if the patient has superficial, herpetic keratitis or corneal ulcers and wounds;
- if the patient has tuberculosis of the eyeball;
- if the patient has a fungal eye infection;
- if the patient has glaucoma with a narrow and open angle of filtration.
Warnings and precautions
Before starting to use Dexamytrex, you should discuss it with your doctor or pharmacist,
especially in the case of:
- if the patient is using other eye drops;
- if the patient is using contact lenses. It is not recommended to use contact lenses while using Dexamytrex.
You should contact your doctor immediately if, while using Dexamytrex, the following occur:
- partial or complete hearing loss or dizziness;
- an increase in the amount of purulent discharge, inflammation, or pain;
- a sudden, very severe eye and head pain with accompanying nausea, vomiting, sudden blurring of vision, and decreased visual acuity. These may be symptoms of increased pressure in the eyeball;
- blurred vision or other vision disturbances;
- blurred vision and difficulty seeing in bright light. These may be symptoms of a disease called cataracts;
- if any other eye problems occur (pain, redness of the eye, tearing, photophobia). These may be symptoms of eye damage.
You should consult your doctor if the patient experiences swelling and weight gain, visible
especially on the torso and face, as these are usually the first symptoms of a disease called Cushing's syndrome. Adrenal insufficiency may occur as a result of discontinuing long-term or intensive use of Dexamytrex. You should consult your doctor before the patient decides to discontinue treatment. This risk is particularly important in children and patients treated with ritonavir or cobicistat.
You should not use the drug for a long time, as gentamicin-resistant bacteria or secondary eye infections (bacterial, fungal, or viral) may develop.
Children and adolescents
The safety and efficacy of Dexamytrex in children have not been established.
Dexamytrex and other drugs
You should tell your doctor about all the drugs the patient is currently taking or has recently taken, including those that are available without a prescription.
In particular, you should tell your doctor if the patient is taking:
- amphotericin B, heparin, sulfadiazine, cefalotin, and cloxacillin, administered topically to the eye. Concurrent administration of Dexamytrex with any of these drugs may lead to the formation of visible precipitates in the conjunctival sac;
- other eye drops or ointments. If Dexamytrex is used at the same time as other eye drops or ointments, a 15-minute interval should be maintained between the administration of the drugs. Eye ointments should always be used last;
- ritonavir or cobicistat, as these drugs may increase the dexamethasone content in the blood.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this drug.
Dexamytrex should not be used during pregnancy and breastfeeding.
Driving and using machines
Even when used as recommended, Dexamytrex may temporarily disturb vision after application, so you should not drive or operate machines until the symptoms have completely resolved.
Dexamytrex contains disodium phosphate dodecahydrate and potassium dihydrogen phosphate
The drug contains 0.20 mg of phosphates in each drop, which corresponds to 6.52 mg/ml.
In patients with severe damage to the transparent, anterior part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding during treatment due to calcium deposition.
3. How to use Dexamytrex
This drug should always be used as directed by the doctor. In case of doubts, you should consult a doctor or pharmacist.
Recommended dose
If not otherwise prescribed, 1 drop into the conjunctival sac of the infected eye 4-6 times a day.
Pull down the lower eyelid and instill 1 drop into the conjunctival sac.
The treatment duration should not be longer than 2 weeks.
Warning!
Administration instructions
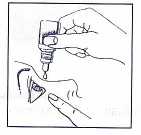
Before instilling the drug, you should wash your hands thoroughly.
Tilt your head back and gently pull down the lower eyelid with your index finger. With the other hand, hold the bottle vertically over the eye, without touching the eye, and instill one drop into the conjunctival sac by gently squeezing the bottle wall.
You should try to keep the eye open and move it so that the solution is evenly distributed. Then, you should gently press the area of the eye corner, on the side of the nose, for 2 minutes. This can help limit the absorption of the drug into the entire body.
Dexamytrex eye drops are sterile. To avoid contaminating the eye drops, you should not touch the dropper tip with your fingers or touch it to the eye surface or any other surface. Using contaminated drops can lead to serious eye damage, including vision loss.
After using the drug, you should put on the protective cap and carefully close the bottle.
If you feel that the effect of Dexamytrex is too strong or too weak, you should consult a doctor or pharmacist.
Warning:
If Dexamytrex is used at the same time as other eye drops or ointments, a 15-minute interval should be maintained between the administration of the drugs. Eye ointments should always be used last.
Using a higher dose of Dexamytrex than recommended
Since the drug is used for the eyes, you should not expect an overdose.
Missing a dose of Dexamytrex
You should take the missed dose as soon as possible. You should not take a double dose to make up for the missed dose. If you have missed several doses, you should inform your doctor and follow their instructions.
4. Possible side effects
Like all drugs, Dexamytrex can cause side effects, although not everybody gets them.
The frequency of possible side effects is defined as follows:
Very common - occurs in more than 1 in 10 patients.
Common - occurs in 1 to 10 in 100 patients.
Uncommon - occurs in 1 to 10 in 1,000 patients.
Rare - occurs in 1 to 10 in 10,000 patients.
Very rare - occurs in less than 1 in 10,000 patients.
Unknown frequency - cannot be estimated from the available data.
Possible side effects:
Rare:
Mild, transient, and short-term vision disturbances.
Very rare:
Pupil dilation in the treated eye.
Unknown frequency:
Difficulty in wound healing (if the drug is used after corneal injuries), cataracts (after long-term use), glaucoma (after long-term use), eye irritation, deposits in the cornea, hypersensitivity (including eyelid and conjunctival edema, itching, conjunctival hyperemia, contact dermatitis).
Fungal keratitis developing particularly easily as secondary infections during long-term local use of corticosteroid-containing drugs. After using corticosteroids, you should always consider the possibility of fungal infection if a persistent corneal ulcer occurs.
Bacterial eye infection.
Secondary infections with pathogens: bacterial, viral (including herpes simplex virus).
Increased intraocular pressure (which may be associated with optic nerve damage, decreased visual acuity, and visual field defects).
Subcapsular cataract.
Perforation (perforation) of the membrane on the surface of the eye.
Hormonal disorders: increased hair growth on the body (especially in women), muscle weakness, and muscle mass loss, purple striae on the skin, increased blood pressure, irregular menstrual periods or amenorrhea, changes in protein and calcium levels in the body, growth retardation in children and adolescents, and swelling and weight gain, visible especially on the torso and face (Cushing's syndrome) (see section 2 "Warnings and precautions").
Blurred vision.
Other side effects reported in connection with the use of eye drops containing phosphates.
In patients with severe damage to the transparent, anterior part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding during treatment due to calcium deposition.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help gather more information on the safety of the drug.
5. How to store Dexamytrex
The drug should be stored out of sight and reach of children.
You should not use this drug after the expiry date stated on the packaging. The expiry date refers to the last day of the given month.
Store in a temperature below 25°C. Store in the original packaging to protect from light.
You should discard the remaining drops after 30 days from the first opening of the bottle.
Drugs should not be disposed of in the sewage system or household waste containers. You should ask your pharmacist how to dispose of unused drugs. This will help protect the environment.
6. Contents of the packaging and other information
What Dexamytrex contains
- The active substances of the drug are: gentamicin (in the form of gentamicin sulfate) and dexamethasone (in the form of dexamethasone sodium phosphate). 1 ml of the drops contains 5 mg of gentamicin sulfate and 1 mg of dexamethasone sodium phosphate.
- The other ingredients are: cetrimide, disodium phosphate dodecahydrate, potassium dihydrogen phosphate, sodium metabisulfite, glycerol 85%, povidone K 25, hypromellose, disodium edetate, water for injections.
What Dexamytrex looks like and what the packaging contains
Dexamytrex is available in the form of eye drops, solution.
LDPE bottle with LDPE dropper and HDPE cap, in a cardboard box.
Pack size: 5 ml.
For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Portugal, the country of export:
Angelini Pharma Portugal, Unipessoal Lda
Rua João Chagas, 53 – Piso 3
1499-040 Cruz Quebrada-Dafundo
Portugal
Manufacturer:
Dr. Gerhard Mann Chem.-Pharm. Fabrik GmbH
Brunsbütteler Damm 165-173
13581 Berlin
Germany
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Authorization number in Portugal, the country of export:8707208
Parallel import authorization number:313/13
Date of leaflet approval: 07.04.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Bausch + Lomb Ireland limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DexamitrexDosage form: Drops, (3 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Rafarm S.A.Prescription requiredDosage form: Drops, (5 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription requiredDosage form: Ointment, (5 mg + 0.3 mg)/gActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription required
Alternatives to Dexamitrex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Dexamitrex in Ukraine
Alternative to Dexamitrex in Spain
Online doctors for Dexamitrex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Dexamitrex – subject to medical assessment and local rules.














