
Ciclo 3 Fort

Ask a doctor about a prescription for Ciclo 3 Fort

How to use Ciclo 3 Fort
Leaflet attached to the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
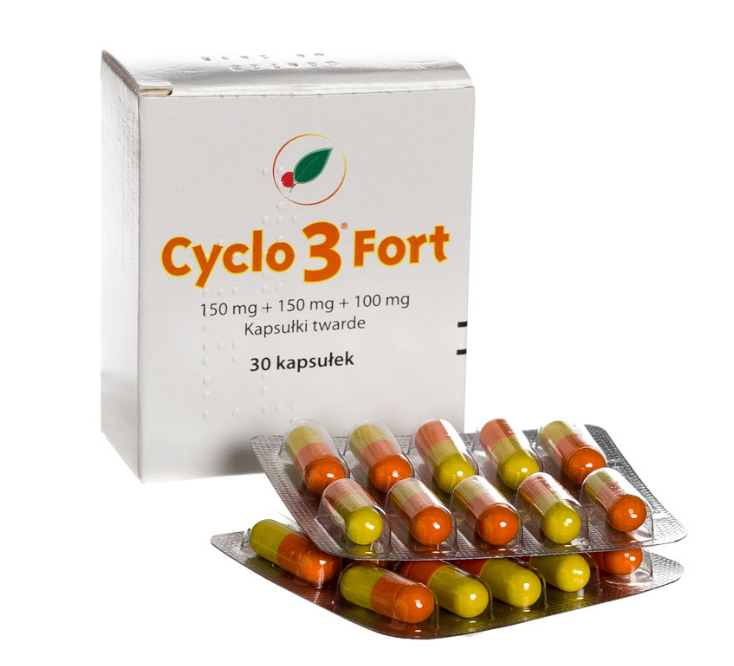
Cyclo 3 Fort
150 mg + 150 mg + 100 mg, hard capsules
Dry extract of Ruscus aculeatus L., Rhizoma (butcher's broom root) + Hesperidin methylchalcone + Ascorbic acid (Vitamin C)
It is necessary to carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
This medicine should always be taken exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If there is no improvement or the patient feels worse after 7 days of taking the medicine for symptoms related to hemorrhoids or after 14 days for symptoms related to venous insufficiency, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Cyclo 3 Fort and what is it used for
- 2. Important information before taking Cyclo 3 Fort
- 3. How to take Cyclo 3 Fort
- 4. Possible side effects
- 5. How to store Cyclo 3 Fort
- 6. Package contents and other information
1. What is Cyclo 3 Fort and what is it used for
Cyclo 3 Fort is a combination of three active substances: dry extract of Ruscus aculeatus L., Rhizoma (butcher's broom root), hesperidin methylchalcone, and ascorbic acid (Vitamin C).
Cyclo 3 Fort belongs to a group of medicines that affect venous elasticity. This medicine has a toning and protective effect on blood vessels - it improves venous wall tension, strengthens capillaries, and reduces blood vessel permeability.
Cyclo 3 Fort is used to treat symptoms related to venous insufficiency (feeling of heaviness in the lower limbs, pain) and as an adjunct in the treatment of hemorrhoids (piles).
Cyclo 3 Fort is intended for adult patients.
2. Important information before taking Cyclo 3 Fort
When not to take Cyclo 3 Fort
- if the patient is allergic to the active substances (dry extract of Ruscus aculeatus L., Rhizoma, hesperidin methylchalcone, ascorbic acid) or any of the other ingredients of this medicine (listed in section 6),
- in case of iron metabolism disorders (thalassemia, hemochromatosis, sideroblastic anemia) due to the presence of ascorbic acid (Vitamin C) in the composition of the medicine.
Warnings and precautions
Before starting treatment with Cyclo 3 Fort, the patient should discuss it with their doctor or pharmacist.
If skin inflammation or subcutaneous thickening or skin ulcers occur, the patient should contact their doctor.
If sudden swelling of one or both lower limbs, heart failure, or kidney failure occurs, the patient should contact their doctor.
If diarrhea occurs, treatment should be discontinued and the doctor contacted (see section 4).
Venous circulation disorders: if discomfort and/or vessel fragility do not improve after 2 weeks of treatment, the patient should contact their doctor.
Hemorrhoid-related disorders: taking Cyclo 3 Fort cannot replace specialized treatment of other anal conditions. If hemorrhoids persist after a few days of treatment (no longer than a week), a medical consultation is necessary.
Ascorbic acid (Vitamin C) may affect the results of certain laboratory tests, such as glucose, bilirubin, aminotransferase, lactate dehydrogenase, and other indicators.
Children and adolescents
This medicine should not be used in children and adolescents.
Cyclo 3 Fort and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Cyclo 3 Fort contains ascorbic acid. Caution should be exercised when using it with deferoxamine and deferiprone (medicines used in particular for diseases caused by excess iron or aluminum in the body).
No studies have been conducted on interactions with other medicines.
Cyclo 3 Fort with food, drink, and alcohol
No studies have been conducted on interactions with food, drink, and alcohol.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
As a precaution, Cyclo 3 Fort should be avoided during pregnancy.
The medicine should not be taken during breastfeeding.
There is no data on the effect of the medicine on fertility.
Driving and using machines
No studies have been conducted on the effect of Cyclo 3 Fort on the ability to drive and use machines.
Cyclo 3 Fort containsorange yellow FCF (E 110). The medicine may cause allergic reactions.
3. How to take Cyclo 3 Fort
This medicine should always be taken exactly as described in the patient leaflet or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
The medicine is indicated for use in adults.
For the treatment of symptoms related to venous insufficiency (feeling of heaviness in the lower limbs, pain), it is recommended to take Cyclo 3 Fort in a dose of 2 to 3 capsules per day.
The patient can take one capsule in the morning and one in the evening or two capsules in the morning and one in the evening.
If symptoms persist for more than 2 weeks despite taking the medicine, the patient should contact their doctor or pharmacist.
As an adjunct in the treatment of hemorrhoid-related symptoms, Cyclo 3 Fort is used in a dose of 4 to 5 capsules per day. The patient can take two capsules in the morning and two in the evening or three capsules in the morning and two in the evening.
For the treatment of hemorrhoid-related symptoms, the medicine is intended for short-term use. If symptoms persist after a few days of treatment (no longer than a week), the patient should contact their doctor.
The medicine should be taken orally.
The capsules should be swallowed with a glass of water.
Use in children and adolescents
The medicine is indicated for use in adults.
Taking a higher dose of Cyclo 3 Fort than recommended
Excessive doses of ascorbic acid (Vitamin C), which is one of the ingredients of the medicine, may cause hemolytic anemia in people with a deficiency of the enzyme glucose-6-phosphate dehydrogenase (G6PD).
Taking ascorbic acid in a dose higher than 1g per day may cause the formation of kidney stones (formation of deposits in the urinary tract made of calcium oxalate crystals).
Missing a dose of Cyclo 3 Fort
A double dose should not be taken to make up for a missed dose.
If the patient has any further doubts about the use of this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Cyclo 3 Fort can cause side effects, although not everybody gets them.
Side effects that may occur:
- Frequent(occurring in less than 1 in 10 people): Diarrhea, sometimes severe, quickly resolving after treatment discontinuation (see section 2. "Warnings and precautions"). Abdominal pain.
- Uncommon(occurring in less than 1 in 100 people): Insomnia (difficulty sleeping). Gastrointestinal disorders. Nausea. Rash (skin redness). Itching. Muscle cramps. Limb pain (arms and/or legs).
- Rare(occurring in less than 1 in 1,000 people): Nervousness. Dizziness.
Feeling of cold in the limbs.
Pain in the veins (venous sensitivity).
Gastrointestinal disorders.
Aphthous stomatitis (gingivitis with the presence of vesicles).
Increased activity of alanine aminotransferase (liver enzyme).
- Unknown frequency(frequency cannot be estimated from available data): Stomach pain. Maculopapular rash (skin redness) and urticaria (itchy, swollen skin redness that may have a blister-like appearance). Reversible microscopic, mainly lymphocytic colitis in some cases (or in some patients).
Ascorbic acid affects the results of some laboratory tests.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl
Reporting side effects can help gather more information on the safety of the medicine.
5. How to store Cyclo 3 Fort
The medicine should be stored out of sight and reach of children.
Store in a temperature below 25°C.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste containers. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Cyclo 3 Fort contains
The active substances of the medicine are:
150 mg of dry extract of Ruscus aculeatus L., Rhizoma (butcher's broom root) (5-7.5:1)
Solvent: ethanol 85% (V/V)
Hesperidin methylchalcone 150 mg
Ascorbic acid (Vitamin C) 100 mg
Other ingredients are:
Capsule contents: talc, magnesium stearate, colloidal hydrophobic silica, macrogol 6000.
Capsule shell composition:
Body (yellow) - quinoline yellow (E 104), orange yellow FCF (E 110), titanium dioxide (E 171), gelatin.
Capsule (orange) - orange yellow FCF (E 110), titanium dioxide (E 171), gelatin.
What Cyclo 3 Fort looks like and what the package contains
Hard capsule, consisting of an opaque yellow body and an opaque orange cap.
30 or 60 capsules in a cardboard box.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Romania, the country of export:
Pierre Fabre Medicament, Les Cauquillous, 81500 Lavaur, France
Manufacturer:
Pierre Fabre Medicament Production, Etablissement Progipharm, Rue du Lycee B.P. 77, 45502 Gien, Cedex, France
Parallel importer:Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Romanian marketing authorization number, country of export: 7293/2006/01
Parallel import authorization number: 232/24
Date of leaflet approval: 10.06.2024
[Information about the trademark]
- Country of registration
- Prescription requiredNo
- Marketing authorisation holder (MAH)Pierre Fabre Medicament
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ciclo 3 FortDosage form: Tablets, 1000 mgActive substance: diosminManufacturer: Polfarmex S.A.Prescription not requiredDosage form: Tablets, 100 mg + 25 mgActive substance: rutoside, combinationsPrescription not requiredDosage form: Tablets, 1000 mgActive substance: diosminPrescription not required
Online doctors for Ciclo 3 Fort
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ciclo 3 Fort – subject to medical assessment and local rules.














