
Cosopt Pf Multi

Ask a doctor about a prescription for Cosopt Pf Multi

How to use Cosopt Pf Multi
Package Leaflet: Information for the User
Cosopt PF Multi, 20 mg/ml + 5 mg/ml, eye drops, solution
Dorzolamide + Timolol
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. See section 4.
Contents of the Package Leaflet
- 1. What is Cosopt PF Multi and what is it used for
- 2. Important information before using Cosopt PF Multi
- 3. How to use Cosopt PF Multi
- 4. Possible side effects
- 5. How to store Cosopt PF Multi
- 6. Contents of the pack and other information
1. What is Cosopt PF Multi and what is it used for
Cosopt PF Multi contains two active substances: dorzolamide and timolol.
- Dorzolamide belongs to a group of medicines called "carbonic anhydrase inhibitors".
- Timolol belongs to a group of medicines called "beta-adrenergic receptor blockers" (beta-adrenolytics). These medicines lower intraocular pressure by two different mechanisms.
Cosopt PF Multi is indicated for the treatment of glaucoma to lower elevated intraocular pressure, when beta-adrenergic blocking agents alone are not sufficient.
2. Important information before using Cosopt PF Multi
When not to use Cosopt PF Multi
- if you are allergic to dorzolamide hydrochloride, timolol maleate, or any of the other ingredients of this medicine (listed in section 6);
- if you currently have or have a history of respiratory problems, such as asthma or severe chronic obstructive pulmonary disease (a serious lung disease that may cause wheezing, breathing difficulties, and/or a long-lasting cough);
- if you have a slow heart rate, heart failure, or irregular heart rhythm;
- if you have severe kidney disease or severe kidney function disorders, or a history of kidney stones;
- if you have excessive acidification of the blood due to an accumulation of chloride ions in the body (hyperchloremic acidosis).
In case of doubt, consult your doctor or pharmacist before using this medicine.
Warnings and precautions
Before starting treatment with Cosopt PF Multi, discuss with your doctor if you have or have had:
- ischemic heart disease (symptoms include chest pain or tightness, shortness of breath, or feeling of suffocation), heart failure, low blood pressure;
- irregular heart rhythm, such as slow heart rate;
- breathing problems, asthma, or chronic obstructive pulmonary disease;
- circulation problems (such as Raynaud's disease or Raynaud's syndrome);
- diabetes, as timolol may mask the signs and symptoms of hypoglycemia (low blood sugar);
- hyperthyroidism, as timolol may mask its signs and symptoms.
- any allergies or anaphylactic reactions
- muscle weakness or diagnosed myasthenia gravis.
- if you wear soft contact lenses, as no studies have been conducted on the use of Cosopt PF Multi in patients wearing contact lenses.
Patient with a history of silver hypersensitivity should not use this medicinal product, as the drops may contain trace amounts of silver from the container.
Before surgery, inform your doctor that you are using Cosopt PF Multi, as timolol may affect the action of certain anesthetics.
After administration to the eye, Cosopt PF Multi may cause systemic effects.
During treatment with Cosopt PF Multi, inform your doctor:
- if you experience eye irritation or any new eye problems, such as redness of the eye or swelling of the eyelids
- if you suspect that Cosopt PF Multi is causing an allergic reaction or hypersensitivity (e.g., skin rash, severe skin reaction, or redness and itching of the eye), discontinue use of this medicine and contact your doctor immediately.
- if you experience eye infection, eye injury, eye surgery, or a reaction that is accompanied by the appearance of new or worsening symptoms.
Children and adolescents
Experience with COSOPT (preservative-containing formulation) in infants and children is limited.
Use in elderly patients
In studies conducted with COSOPT (preservative-containing formulation), the effects were similar in elderly and younger patients.
Use in patients with liver function disorders
Inform your doctor about any current or past liver diseases.
Cosopt PF Multi and other medicines
Cosopt PF Multi may affect the action of other medicines or other medicines you are taking may affect the action of Cosopt PF Multi. This includes other ophthalmic glaucoma medicines.
Tell your doctor or pharmacist about any medicines you are taking or plan to take, including those for lowering blood pressure, heart medicines, or diabetes medicines.
This is especially important for:
- taking medicines for lowering blood pressure or heart diseases (such as calcium channel blockers, beta-adrenergic blockers, or digoxin);
- taking medicines for irregular heart rhythms or restoring a regular heart rhythm (such as calcium channel blockers, beta-adrenergic blockers, or digoxin);
- using other eye drops containing beta-adrenergic blockers;
- taking other carbonic anhydrase inhibitors, such as acetazolamide;
- taking monoamine oxidase inhibitors (MAOIs);
- taking parasympathomimetic (cholinergic) medicines, which may be used for urinary disorders. Parasympathomimetic medicines are also sometimes used to restore normal bowel motility;
- taking opioid medicines, such as morphine, used for moderate to severe pain;
- taking diabetes medicines;
- taking antidepressant medicines, such as fluoxetine and paroxetine;
- taking sulfonamide chemotherapeutics;
- taking quinidine (a medicine used for heart diseases and certain types of malaria).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Use during pregnancy
Do not use Cosopt PF Multi during pregnancy unless your doctor considers it necessary.
Use during breastfeeding
Do not use Cosopt PF Multi during breastfeeding. Timolol may pass into breast milk.
Driving and using machines
No studies have been conducted on the effects of Cosopt PF Multi on the ability to drive or operate machinery.
Cosopt PF Multi can cause side effects such as blurred vision, which may affect the ability to drive or operate machinery.
Patients who experience side effects such as blurred vision should not drive or operate machinery.
3. How to use Cosopt PF Multi
Always use this medicine exactly as your doctor has told you.
The recommended dose is one drop in the affected eye(s) in the morning and evening.
If you are using other eye drops, wait at least 10 minutes between administering each medicine.
Do not change the dose of your medicine without consulting your doctor.
If you have difficulty administering the drops, ask a family member or caregiver for help.
Do not allow the tip of the multidose container to come into contact with the eye or its surroundings.
This can cause eye injury and contamination of the container with bacteria, which can lead to serious eye damage and vision loss.
To avoid possible contamination of the multidose container, wash your hands before using this medicine and avoid touching the tip of the container to any surface.
Instructions for use
Before administering the eye drops:
- Wash your hands before opening the bottle.
- Do not use the medicine if the protective ring around the neck of the bottle is damaged before the first use.
- Before administering the drops to the eye, try using the bottle with the dropper by slowly squeezing it until one drop is released from the bottle, away from the eye.
- If you are sure you can administer a single drop, take the most comfortable position for administration (you can sit, lie on your back, or stand in front of a mirror).
- When opening the bottle for the first time, discard the first drop of medicine away from the eye.
Administering the drops:
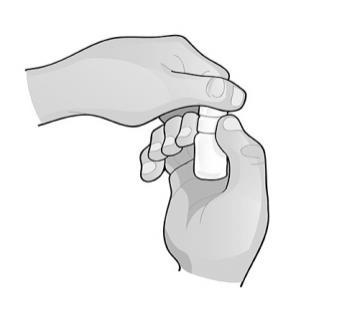
- 1. Hold the bottle directly under the cap and open it by twisting the cap. Do not touch the dropper tip to any surface to avoid contaminating the solution.
- 2. Tilt your head back and hold the bottle above the eye.
- 3. Pull the lower eyelid down and look up. Gently squeeze the bottle until the drop falls into the eye. Remember that it may take a few seconds from squeezing the bottle to the drop appearing. Do not squeeze too hard.
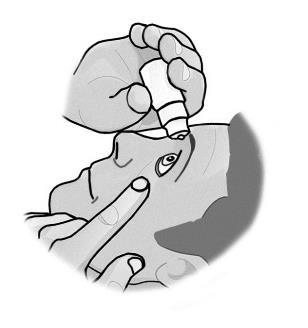
- 4. Close your eye and press the inner corner of your eye with your finger for about two minutes. This helps prevent the medicine from entering the entire body.
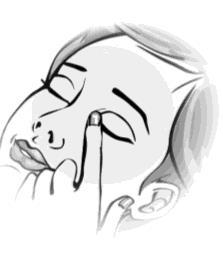
- 5. To administer the drops to the other eye, if instructed by your doctor, repeat steps 2 to 4. Consult your doctor about how to use this medicine, as sometimes only one eye needs treatment.
- 6. After each use, shake the bottle downwards to remove any remaining solution from the tip of the bottle. Do not touch the tip of the bottle. This is necessary to ensure further administration.
- 7. Wipe off any excess solution from the skin around the eyes.
- 8. After 2 months of using Cosopt PF Multi, a small amount of solution will remain in the bottle. Do not attempt to use the remaining medicine. Do not use the medicine for more than 2 months after opening the bottle.
If you have any doubts about how to administer the medicine, ask your doctor, pharmacist, or nurse.
Using more Cosopt PF Multi than prescribed
If you accidentally administer too many drops or swallow the contents of the container, among other symptoms, you may experience dizziness, difficulty breathing, or a feeling of slow heart rate. Contact your doctor immediately.
Missing a dose of Cosopt PF Multi
Cosopt PF Multi should be used as directed by your doctor.
If you miss a dose, use it as soon as possible. However, if it is almost time for your next dose, do not use the missed dose and return to your regular dosing schedule.
Do not use a double dose to make up for a missed dose.
Stopping treatment with Cosopt PF Multi
Before stopping treatment, consult your doctor.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Cosopt PF Multi can cause side effects, although not everybody gets them.
Side effects:
If you experience any of the following side effects, stop using this medicine and seek medical attention immediately, as they may be signs of an allergic reaction.
Rare (may affect up to 1 in 1000 people):
- Chest pain, swelling (fluid accumulation), changes in heart rhythm or rate, congestive heart failure (a heart disease characterized by shortness of breath and swelling of the feet and legs due to fluid accumulation), cardiac arrest, heart block, low blood pressure, cerebral ischemia (reduced blood flow to the brain), stroke.
- Shortness of breath, respiratory failure, bronchospasm.
- Systemic allergic reactions, including anaphylaxis, angioedema, urticaria, pruritus, rash.
- Severe skin reactions, including Stevens-Johnson syndrome.
Other side effects:
Treatment with eye drops can usually be continued, unless the side effects are severe. If you are concerned, consult your doctor or pharmacist.
Do not stop using Cosopt PF Multi without consulting your doctor.
The following side effects have been reported during clinical trials or after the marketing of Cosopt PF Multi or one of its active substances:
Very common (may affect more than 1 in 10 people)
Burning and stinging in the eye, change in taste.
Common (may affect up to 1 in 10 people)
- Ocular side effects: conjunctival injection, eye irritation (including stinging, burning, itching, tearing, and redness), corneal erosions, ocular pain, blurred vision, dry eye.
- General side effects: headache, sinusitis, nausea, fatigue.
Uncommon (may affect up to 1 in 100 people)
- Ocular side effects: uveitis, visual disturbances, including refractive changes (in some cases due to the discontinuation of miotic therapy).
- General side effects: dizziness, depression, bradycardia, syncope, dyspnea, epistaxis, gastrointestinal disorders.
Rare (may affect up to 1 in 1000 people)
- Ocular side effects: transient myopia, which may be reversible upon discontinuation of treatment, choroidal detachment (a separation of the layer of blood vessels between the sclera and retina), eyelid oedema (swelling of the eyelids), double vision, eye discharge, corneal oedema (with symptoms of blurred vision), decreased intraocular pressure.
- General side effects: tachycardia (fast or irregular heartbeat), Raynaud's phenomenon, peripheral vasospasm (cold hands and feet), decreased peripheral circulation, muscle cramps and leg pain (claudication), cough, throat irritation, dry mouth, insomnia, nightmares, memory loss, paresthesia (tingling or numbness of hands or feet), exacerbation of myasthenia gravis (a muscle disorder), decreased libido, systemic lupus erythematosus (an autoimmune disease that can cause inflammation of internal organs), tinnitus (ringing in the ears), sinusitis, epistaxis, diarrhea, contact dermatitis, alopecia, psoriasiform rash, Peyronie's disease (a condition that can cause curvature of the penis), allergic reactions, such as rash, urticaria, pruritus, in rare cases also angioedema, eyelid oedema, and mouth oedema, wheezing.
As with other eye drops, timolol is absorbed into the bloodstream, which may cause side effects similar to those observed with oral beta-adrenergic blockers.
These side effects occur less frequently with eye drops than with oral or injected beta-adrenergic blockers.
Unknown frequency (cannot be estimated from the available data)
Hypoglycemia, abdominal pain, vomiting, muscle pain unrelated to physical exertion, sexual dysfunction, hallucinations, foreign body sensation in the eye, tachycardia and increased blood pressure.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist.
Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the Marketing Authorization Holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Cosopt PF Multi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and bottle after EXP.
The expiry date refers to the last day of the month stated.
Do not store above 25°C.
After opening the bottle, the solution can be used for 2 months.
The bottle must be kept tightly closed.
Do not use the medicinal product if the container's seal is damaged.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required.
This will help protect the environment.
6. Contents of the pack and other information
What Cosopt PF Multi contains
- The active substances are dorzolamide and timolol. Each milliliter of solution contains 20 mg of dorzolamide (22.26 mg of dorzolamide hydrochloride) and 5 mg of timolol (6.83 mg of timolol maleate).
- The other ingredients are sodium citrate, hydroxyethylcellulose, mannitol, sodium hydroxide (to adjust pH), and water for injections.
What Cosopt PF Multi looks like and contents of the pack
Cosopt PF Multi is a clear, colorless or almost colorless, slightly viscous solution, practically free from visible particles.
The solution is contained in a white plastic bottle with a white dropper and a white protective cap.
Pack sizes: 1, 2, or 3 bottles, each containing 10 ml of solution, in a carton box.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Tubilux Pharma S.P.A.
Via Costarica 20/22
00071 Pomezia (Roma)
Italy
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Date of last revision of the package leaflet:
| Bulgaria, Croatia, Cyprus, Greece, United Kingdom (Northern Ireland) | COSOPT iMulti |
| Belgium, Luxembourg | COSOPT Sine Conservans |
| Czech Republic | COSOPT bez konzervačních přísad |
| Denmark | Cosopt iMulti ukonserveret |
| Sweden | Cosopt sine |
| Finland, Lithuania, Iceland, Germany, Norway | COSOPT sine |
| Hungary, Portugal | COSOPT Multi |
| Italy | COSOPT senza conservante |
| Latvia, Spain | COSOPT PF |
| Poland | Cosopt PF Multi |
| Romania | COSOPT fără conservant |
| Slovakia | COSOPT Multi Dose Free |
| Slovenia | COSOPT brez konzervansa |
| France | COSTEC |
| Netherlands | COSOPT Multidose conserveermiddelvrij |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSanten OY Tubilux Pharma S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Cosopt Pf MultiDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Cosopt Pf Multi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cosopt Pf Multi in Испания
Alternative to Cosopt Pf Multi in Украина
Online doctors for Cosopt Pf Multi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cosopt Pf Multi – subject to medical assessment and local rules.














