
Combigan

Ask a doctor about a prescription for Combigan

How to use Combigan
Leaflet accompanying the packaging: patient information
Combigan, 2 mg/ml + 5 mg/ml, eye drops, solution
Brimonidine tartrate + Timolol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Combigan and what is it used for
- 2. Important information before using Combigan
- 3. How to use Combigan
- 4. Possible side effects
- 5. How to store Combigan
- 6. Contents of the packaging and other information
1. What is Combigan and what is it used for
Combigan eye drops are used to treat glaucoma. They contain two active substances (brimonidine and timolol) that lower high pressure in the eye. Brimonidine belongs to a group of medicines called alpha-2 adrenergic receptor agonists. Timolol belongs to a group of medicines called beta-adrenergic blockers. Combigan is prescribed to treat high pressure in the eye when using eye drops containing only a beta-adrenergic blocker is not sufficient.
The eye contains a clear, watery fluid that fills its interior and performs nutritional functions. This fluid is constantly being removed from the eye and replaced with newly produced fluid. If the fluid is not removed from the eye quickly enough, the pressure inside the eye increases, which can lead to vision damage. Combigan works by reducing the production of fluid and increasing the amount of fluid removed from the eye. This reduces the pressure in the eye while still nourishing it.
2. Important information before using Combigan
When not to use Combigan
A patient who may be affected by any of the above points should not use Combigan until they have consulted their doctor again.
Warnings and precautions
Before starting to use Combigan, the patient should discuss the following with their doctor:
- the occurrence of any of the following conditions, currently or in the past:
- coronary heart disease (symptoms may include chest pain or feeling of suffocation), heart failure, low blood pressure
- irregular heart rhythm, including slow heart rate
- respiratory problems, asthma, severe chronic obstructive pulmonary disease
- diseases with peripheral circulation disorders (such as Raynaud's phenomenon)
- diabetes, as timolol may mask the symptoms of low blood sugar
- hyperthyroidism, as timolol may mask its symptoms
- kidney or liver disease
- adrenal gland tumor
- surgical procedures on the eye to reduce pressure in the eyeball
- current or past allergies (e.g., hay fever, rash) or severe allergic reactions; it should be remembered that in such cases, it may be necessary to increase the dose of adrenaline in the treatment of a potential severe allergic reaction
- the patient should inform the anesthesiologist about the use of Combigan, as timolol may affect the action of certain medicines during general anesthesia.
Children and adolescents
The medicine should not be given to children under 2 years of age, and it is not recommended for use in children and adolescents (from 2 to 17 years of age).
Combigan and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those used for other conditions, and those available without a prescription.
Combigan may affect the action of other medicines taken at the same time, including eye drops used to treat glaucoma. On the other hand, these medicines may affect the action of Combigan.
There are many medicines that can interact with Combigan, so it is especially important to inform the doctor if the patient is taking:
- painkillers
- sleeping pills or sedatives
- high blood pressure medicines
- heart medicines (e.g., for arrhythmias) such as beta-adrenergic blockers, digoxin, quinidine (used to treat heart conditions and some types of malaria)
- medicines for diabetes or conditions with high blood sugar levels
- antidepressant medicines, such as fluoxetine or paroxetine
- other eye drops used to lower high blood pressure in the eye (glaucoma)
- medicines used to treat severe allergic reactions
- medicines that affect the level of certain hormones in the body, such as adrenaline, dopamine
- medicines that affect the muscles in blood vessels
- medicines for heartburn or stomach ulcers
The patient should inform their doctor if the dose of any recently taken medicine has changed, and if they regularly consume alcohol.
If the patient is to undergo anesthesia, they should inform their doctor or dentist about the use of Combigan.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Combigan should not be used in pregnant women, except in cases where the doctor considers it necessary.
Combigan should not be used during breastfeeding. Timolol may pass into breast milk. During breastfeeding, before using any medicine, the patient should consult their doctor.
Driving and using machines
In some patients, Combigan may cause drowsiness, fatigue, or blurred vision. The patient should not drive vehicles or operate machines until these symptoms have resolved. If problems persist, the patient should consult their doctor.
Combigan contains benzalkonium chloride
This medicine contains 0.25 mg of benzalkonium chloride in every 5 ml of solution, which corresponds to 0.05 mg/ml.
- Benzalkonium chloride is a preservative that can be absorbed by soft contact lenses and may change their color. Before using this medicine, the patient should remove their contact lenses and wait 15 minutes before putting them back on.
- Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer on the front of the eye). If discomfort, stinging, or pain in the eye occurs after using this medicine, the patient should contact their doctor.
Combigan contains phosphates
This medicine contains 52.9 mg of phosphates in every 5 ml of solution, which corresponds to 10.58 mg/ml.
If the patient has severe damage to the transparent layer on the front of the eye (cornea), phosphates may rarely cause cloudy spots on the cornea during treatment due to calcium deposits.
3. How to use Combigan
Combigan should always be used exactly as prescribed by the doctor. If the patient has any doubts, they should consult their doctor or pharmacist.
Combigan should not be used in young children under 2 years of age. Combigan is also not recommended for use in children and adolescents (from 2 to 17 years of age).
The recommended dose of Combigan is one drop twice a day, with an interval of about 12 hours. The patient should not change the dose or stop using the medicine without consulting their doctor.
If Combigan is used with other eye drops, the patient should wait at least 5 minutesbetween instilling Combigan and administering other eye drops.
Method of administration
The patient should not use the bottle if the protective seal on the neck of the bottle is damaged before the first use.
Before opening the bottle, the patient should wash their hands thoroughly. Before instilling the drops, the patient should tilt their head back and look up at the ceiling.
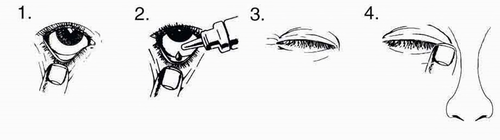
- 1. Gently pull down the lower eyelid to form a small pocket.
- 2. Turn the bottle upside down and gently squeeze it to release one drop into each eye to be treated.
- 3. Release the lower eyelid and close the eye.
- 4. Keep the eye closed and press the corner of the eye (where the eye meets the nose) with their finger for two minutes. This helps reduce the risk of the medicine getting into the body.
If the patient misses a drop, they should try again.
To avoid contaminating the drops, the patient should avoid touching their eye or other surfaces with the dropper tip. Immediately after use, the patient should replace the cap and tighten the bottle.
Using a higher than recommended dose of Combigan
Adults
It is unlikely that problems will occur due to using too much Combigan. The next dose should be instilled at the usual time. If the patient is concerned, they should consult their doctor.
Infants and children
There have been reports of cases of overdose in infants and children taking brimonidine (one of the ingredients of Combigan) for glaucoma. Symptoms included sleepiness, low muscle tone, low body temperature, paleness, and breathing difficulties.
If these symptoms occur, the patient should contact their doctor immediately.
Adults and children
If Combigan is accidentally ingested, the patient should contact their doctor immediately.
Missing a dose of Combigan
If the patient misses a dose of Combigan, they should instill one drop into each eye to be treated as soon as they remember. Then, they should continue using the medicine regularly according to the prescribed schedule. The patient should not use a double dose to make up for a missed dose.
Stopping the use of Combigan
Combigan should be used every day to achieve the desired effect.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Combigan can cause side effects, although not everybody gets them.
If the patient experiences any of the following side effects, they should contact their doctor immediately:
- heart failure (e.g., chest pain) or irregular heart rhythm
- increased or decreased heart rate or low blood pressure
The following side effects may occur during treatment with Combigan:
Eye-related side effects:
Very common (may affect more than 1 in 10 people):
- eye redness or feeling of burning in the eye
Common (may affect up to 1 in 10 people):
- stinging or pain in the eye
- allergic reactions of the eye or skin around the eye
- small cracks on the surface of the eye (with or without inflammation)
- swelling, redness, or inflammation of the eyelids
- irritation or feeling of a foreign body in the eye
- itching of the eye and eyelids
- nodules or white spots on the conjunctiva of the eye
- vision disturbances
- tearing
- dry eye
- sticky discharge in the eye
Uncommon (may affect up to 1 in 100 people):
- blurred vision
- conjunctival edema or inflammation
- eye fatigue
- increased sensitivity to light
- eyelid pain
- conjunctival pallor
- conjunctival edema or inflammation
- floaters
Unknown (frequency cannot be estimated from the available data):
- blurred vision
Whole-body side effects:
Common (may affect up to 1 in 10 people):
- high blood pressure
- depression
- drowsiness
- headache
- dry mouth
- general weakness
Uncommon (may affect up to 1 in 100 people):
- heart failure
- irregular heart rhythm
- mild dizziness
- fainting
- dry nose
- taste disturbances
- nausea
- diarrhea
Unknown (frequency cannot be estimated from the available data):
- increased or decreased heart rate
- low blood pressure
- flushing
Some of these symptoms may be caused by an allergic reaction to one of the ingredients of the medicine.
Side effects also occurred when the active substances in Combigan were used separately, and they may potentially occur when using Combigan.
The following side effects were reported when using brimonidine:
- eye inflammation, constricted pupils, sleep disturbances, cold-like symptoms, shortness of breath, gastrointestinal symptoms, nausea, general allergic reactions, skin reactions including redness, swelling of the face, itching, rash, and vasodilation symptoms.
As with other eye medicines, the active substances in Combigan may be absorbed systemically. Due to the absorption of timolol, the same types of side effects may occur as those observed with systemic beta-adrenergic blockers.
Cases of systemic side effects after topical administration to the eye are less common than with oral or injectable administration.
The following side effects include reactions observed with topical beta-adrenergic blockers:
- generalized allergic reactions, including swelling (occurring in the face and limbs, which may reduce airway patency leading to swallowing or breathing difficulties), urticaria (or itchy rash), localized or generalized rash, itching, and severe, life-threatening allergic reactions with rapid onset.
- low blood sugar.
- sleep disturbances (insomnia), nightmares, memory loss, hallucinations.
- stroke, reduced blood flow to the brain, worsening of myasthenia gravis symptoms (a disease with muscle weakness), sensory disturbances (feeling of tingling or numbness).
- corneal inflammation, detachment of the vascular membrane of the eye (the membrane under the retina, containing blood vessels) after filtration procedures, which may lead to vision disturbances, reduced corneal sensitivity, corneal erosion (damage to the front layer of the eyeball), ptosis (the upper eyelid remains half-closed), double vision.
- chest pain, swelling (fluid accumulation), heart rhythm disturbances, heart attack, heart failure.
- Raynaud's phenomenon, cold hands and feet.
- constriction of airways in the lungs (especially in patients with pre-existing lung disease), breathing difficulties, cough.
- gastrointestinal symptoms, stomach pain, vomiting.
- hair loss, skin rash with a white-silver color (rash resembling psoriasis) or exacerbation of psoriasis, rash.
- muscle pain not caused by exercise or physical exertion.
- sexual dysfunction, decreased libido.
- muscle weakness.
Other side effects reported with phosphate-containing eye drops
Very rarely, in some patients with severe corneal damage, phosphate-containing eye drops have been reported to cause corneal calcification during treatment.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Tel.: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects helps to gather more information on the safety of the medicine.
5. How to store Combigan
The medicine should be stored out of sight and reach of children.
The bottle should be stored in the outer carton to protect it from light.
Only one bottle should be used at a time.
Do not use this medicine after the expiry date stated on the carton and bottle label after EXP. The expiry date refers to the last day of the month stated.
The bottle should be discarded after four weeks from the first opening, even if there are still drops left; this helps prevent infections. To remember, the patient should write the date of the first opening of the bottle in the space provided on the carton.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Combigan contains
- The active substances of Combigan are brimonidine tartrate and timolol
- One milliliter of solution contains 2 milligrams of brimonidine tartrate and 5 milligrams of timolol (in the form of timolol maleate)
- The other ingredients are benzalkonium chloride (preservative), sodium dihydrogen phosphate monohydrate, sodium phosphate anhydrous, and purified water, hydrochloric acid or sodium hydroxide (added to adjust the pH of the solution)
What Combigan looks like and contents of the pack
Combigan is a clear, greenish-yellow solution for use as eye drops. It comes in a plastic bottle with a screw cap. Each bottle is filled to half and contains 5 ml of solution. Each pack contains 1 or 3 bottles. Not all pack sizes may be marketed.
Marketing authorization holder
AbbVie Sp. z o.o.
ul. Postępu 21B
02-676 Warsaw
tel. 22 372 78 00
Manufacturer
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Ireland
This medicine is authorized in the Member States of the European Economic Area under the following names:
| Member State | Medicine name |
| Austria, | Combigan 2 mg/ml + 5 mg/ml Augentropfen |
| Belgium | Combigan 2 mg/ml + 5 mg/ml oogdruppels, oplossing |
| Bulgaria | Комбиган 2 mg/ml + 5 mg/ml капки за очи, разтвор Combigan 2 mg/ml + 5 mg/ml eye drops, solution |
| Czech Republic | COMBIGAN 2 mg/ml + 5 mg/ml oční kapky, roztok |
| Croatia | Combigan 2 mg/ml + 5 mg/ml kapi za oko, otopina |
| Denmark | Combigan 2 mg/ml + 5 mg/ml øjendråber, opløsning |
| Estonia | Combigan, 2 mg/5 mg/ml silmatilgad, lahus |
| Finland | Combigan 2 mg/ml + 5 mg/ml silmätipat, liuos |
| France | COMBIGAN 2 mg/ml + 5 mg/ml, collyre en solution |
| Germany | Combigan 2 mg/ml + 5 mg/ml Augentropfen |
| Greece | COMBIGAN οφθαλμικές σταγόνες, διάλυμα, (0,2 + 0,5)% |
| Hungary | COMBIGAN 2 mg/ml+5 mg/ml oldatos szemcsepp |
| Iceland | Combigan 2 mg/ml + 5 mg/ml augndropar, lausn |
| Ireland | Combigan 2 mg/ml + 5 mg/ml eye drops, solution |
| Italy | COMBIGAN 2 mg/ml + 5 mg/ml collirio, soluzione |
| Latvia | Combigan 2 mg/5 mg/ml acu pilieni, šķīdums |
| Lithuania | Combigan 2 mg/5 mg/ml akių lašai (tirpalas) |
| Luxembourg | Combigan 2 mg/ml + 5 mg/ml, collyre en solution |
| Netherlands | Combigan 2 mg/ml + 5 mg/ml, oogdruppels, oplossing |
| Norway | Combigan 2 mg/ml + 5 mg/ml øyedråper, oppløsning |
| Poland | Combigan eye drops, solution, 2 mg/ml + 5 mg/ml |
| Portugal | Combigan 2 mg/ml + 5 mg/ml colírio, solução |
| Romania | Combigan 2 mg/ml + 5 mg/ml picături oftalmice, soluţie |
| Slovakia | COMBIGAN 2 mg/ml + 5 mg/ml očná roztoková instilácia |
| Slovenia | COMBIGAN 2 mg/ml + 5 mg/ml kapljice za oko, raztopina |
| Spain | Combigan 2 mg/ml + 5 mg/ml colirio en solución |
| Sweden | Combigan 2 mg/ml + 5 mg/ml ögondroppar, lösning |
| United Kingdom | Combigan 2 mg/ml + 5 mg/ml eye drops, solution |
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAllergan Pharmaceuticals Ireland
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CombiganDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Combigan in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Combigan in Іспанія
Alternative to Combigan in Україна
Online doctors for Combigan
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Combigan – subject to medical assessment and local rules.














