

Cetraxal Plus

Ask a doctor about a prescription for Cetraxal Plus

How to use Cetraxal Plus
1. What is Cetraxal Plus and what is it Used for
Cetraxal Plus is a Solution for Administration into the Ear. It Contains:
- Ciprofloxacin, an Antibiotic Belonging to the Group Called Fluoroquinolones. Ciprofloxacin Kills Bacteria that Cause Infections,
- Fluocinolone Acetonide, a Corticosteroid with Anti-inflammatory and Analgesic Effects for the Treatment of Swelling and Pain.
Cetraxal Plus is an Ear Drop Solution. It is Used in Adults and Children from 6 Months of Age for the Treatment of Acute Otitis Externa and Otitis Media with Ventilation Tube Drainage, of Bacterial Origin.
2. Important Information Before Using Cetraxal Plus
When not to Use Cetraxal Plus
- If the Patient is Allergic (Hypersensitive) to Ciprofloxacin, or other Quinolones, Fluocinolone Acetonide, or any of the other Ingredients of Cetraxal Plus (see Section 6).
- If the Patient has Viral or Fungal Ear Infections.
Warnings and Precautions
- This Medication Should be Used Exclusively in the Ear. It Should not be Swallowed, Injected, or Inhaled. It Should not be Administered into the Eye.
- If, after Starting Treatment, the Patient Experiences Hives, Skin Rash, or any other Allergic Symptoms (e.g., Sudden Swelling of the Face, Throat, or Eyelids, Difficulty Breathing), Treatment Should be Discontinued and a Doctor Should be Consulted Immediately.
Severe Allergic Reactions may Require Immediate Emergency Treatment.
- The Doctor Should be Informed if Symptoms do not Improve Before the End of Treatment. As with other Antibiotics, Additional Infections may Sometimes Occur, Caused by Organisms Resistant to Ciprofloxacin. In such Cases, the Doctor will Initiate Appropriate Treatment.
- If the Patient Experiences Blurred Vision or other Vision Disorders, a Doctor Should be Consulted.
Use in Children
Due to the Lack of Sufficient Clinical Data on the Use of Cetraxal Plus in Children under 6 Months, Before Administering this Medication to a Child of this Age, a Doctor Should be Consulted.
Cetraxal Plus and other Medications
The Doctor or Pharmacist Should be Informed about all Medications Currently Used by the Patient, as well as those Planned for Use. This also Applies to Medications Available without a Prescription.
Pregnancy and Breastfeeding
No Appropriate and Controlled Studies have been Conducted with Cetraxal Plus in Pregnant Women.
If the Patient is Pregnant or Breastfeeding, or Thinks they may be Pregnant or Plans to Become Pregnant, they Should Inform their Doctor or Pharmacist Before Using the Medication.
Due to the Fact that it has not been Confirmed that Cetraxal Plus Penetrates into Breast Milk, Particular Caution Should be Exercised when Using Cetraxal Plus during Breastfeeding.
Driving and Operating Machines
Given the Form and Route of Administration, Cetraxal Plus does not Affect the Ability to Drive or Operate Hazardous Machinery.
Cetraxal Plus Contains Methyl Parahydroxybenzoate (E 218) and Propyl Parahydroxybenzoate (E 216), which may Cause Allergic Reactions (Delayed Reactions are Possible).
3. How to Use Cetraxal Plus
Cetraxal Plus is Intended Exclusively for Administration into the Ear (Intratympanic).
Cetraxal Plus Should always be Used in Accordance with the Doctor's or Pharmacist's Recommendations. In Case of Doubts, a Doctor or Pharmacist Should be Consulted.
The Recommended Dose for Adults and Children is 6 to 8 Drops into the Affected Ear, Twice a Day, for 7 Days.
Cetraxal Plus can be Used in both Ears only if Recommended by a Doctor.
The Doctor will Determine how Long to Take Cetraxal Plus. To Avoid Recurrence of the Infection, Treatment Should not be Discontinued too Early, even if the Ear Symptoms have Improved.
Method of Administration
The Person who will Administer Cetraxal Plus Should Wash their Hands.
- 1. Warm the Drops by Holding the Bottle in your Hands for a few Minutes, to Avoid Dizziness that may Occur when Administering a Cold Solution into the Ear Canal.
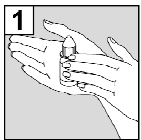
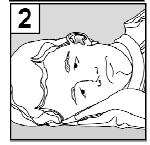
- 2. Tilt your Head to the Side, so that the Affected Ear is Facing Upwards.
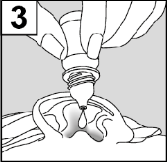
- 3. Introduce the Drops into the Ear using the Dropper. Do not Touch the Dropper to the Ear or Fingers to Avoid Contamination.
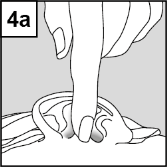
- 4. After Introducing the Drops into the Ear, Follow the Instructions below for the Specific Ear Infection of the Patient: In Patients with Otitis Media with Ventilation Tube Drainage: The Patient Lies on their Side, and the Person Administering Cetraxal Plus Should Gently Press the Skin at the Entrance to the Ear Canal (Figure 4a) 4 Times with a Pumping Motion. This Allows the Drops to Pass through the Drainage in the Eardrum into the Middle Ear. In Patients with Otitis Externa: The Patient Lies on their Side, and the Person Administering Cetraxal Plus Should Gently Pull the Earlobe Upwards and Backwards (Figure 4b). This Allows the Drops to Flow into the Ear Canal.
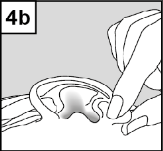
- 5. Keep your Head in the Tilted Position for about 1 Minute to Allow the Medication to Penetrate the Ear.
- 6. If Necessary, the Procedure Should be Repeated for the other Ear.
To Ensure the Effectiveness of the Medication in the Ear, the Administration Instructions Should be Followed. During Administration, it is not Recommended to Keep the Head in an Upright Position or to Move the Head too Suddenly, as this may Result in Loss of Part of the Medication, as the Drops will Flow down the Face instead of Penetrating the Ear Canal.
The Bottle Should be Stored until the End of Treatment. The Medication Should not be Stored for Later Use.
Use of a Higher than Recommended Dose of Cetraxal Plus
No Symptoms of Overdose are Known. In Case of Overdose or Accidental Ingestion of the Medication, a Doctor or Pharmacist Should be Consulted Immediately, or Contact a Poison Control Center by Phone, Providing the Name of the Medication and the Amount Taken, or Go to the Nearest Medical Care Point.
Missed Dose of Cetraxal Plus
A Double Dose Should not be Used to Make up for a Missed Dose. The Next Dose Should be Used According to the Schedule.
Discontinuation of Cetraxal Plus
Cetraxal Plus Should not be Discontinued without Consulting a Doctor or Pharmacist.
It is Very Important to Use these Ear Drops for the Period Indicated by the Doctor, even if the Symptoms Disappear Earlier. If the Medication is Stopped too Early, the Infection may Recur, and the Symptoms may Reappear or even Worsen. Resistance to the Antibiotic may also Develop.
4. Possible Undesirable Effects
Like all Medications, Cetraxal Plus can Cause Undesirable Effects, although they do not Occur in Everyone.
If Severe Allergic Reactions or any of the Following Undesirable Effects Occur, Treatment Should be Discontinued and a Doctor Should be Informed Immediately:
Swelling of the Hands, Feet, Ankles, Face, Lips, Mouth, or Throat, Difficulty Swallowing or Breathing, Rash or Hives, Sores, Ulcers.
Common: May Occur in up to 1 in 10 Patients
Local Undesirable Effects in the Ear: Discomfort, Pain, Itching.
General Undesirable Effects: Taste Disorders.
Uncommon: May Occur in up to 1 in 100 Patients
Local Undesirable Effects in the Ear: Ringing, Residue of the Medication, Blockage of the Drain, Tingling, Redness, Impaired Hearing, Rash, Redness, Fungal Infection of the Outer Ear, Ear Discharge, Swelling, Eardrum Disorders, Granuloma, Middle Ear Infection of the other Ear.
General Undesirable Effects: Fungal Infection with Candida, Irritability, Excessive Tear Production, Dizziness, Redness of the Skin, Headache, Vomiting, Fatigue.
Unknown (Frequency cannot be Estimated from Available Data):
Vision Disorders: Blurred Vision
Reporting of Undesirable Effects
If any Undesirable Effects Occur, including any not Listed in the Leaflet, the Doctor, Pharmacist, or Nurse Should be Informed. Undesirable Effects can also be Reported Directly to the Department of Monitoring of Undesirable Effects of Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C; 02-222 Warsaw, Phone: (+48 22) 49 21 301, Fax: (+48 22) 49 21 309, Website: http://smz.ezdrowie.gov.pl
Undesirable Effects can also be Reported to the Marketing Authorization Holder or Parallel Importer.
By Reporting Undesirable Effects, more Information can be Collected on the Safety of this Medication.
5. How to Store Cetraxal Plus
The Medication Should be Stored in a Place that is not Visible and not Accessible to Children.
Store at a Temperature below 30°C.
Cetraxal Plus Should not be Used after the Expiration Date Stated on the Packaging. The Expiration Date Refers to the Last Day of the Specified Month.
After the First Opening of the Bottle, the Medication Should not be Used for more than 1 Month. After Opening, Store at a Temperature below 25°C.
No Medications Should be Disposed of via Sewer or Household Waste. The Pharmacist Should be Asked what to do with Unused Medications. This will Help Protect the Environment.
6. Contents of the Packaging and other Information
What Cetraxal Plus Contains
- The Active Substances of the Medication are: Ciprofloxacin in the Form of Ciprofloxacin Hydrochloride Monohydrate and Fluocinolone Acetonide. 1 ml of Cetraxal Plus Contains 3 mg of Ciprofloxacin (in the Form of Ciprofloxacin Hydrochloride Monohydrate) and 0.25 mg of Fluocinolone Acetonide.
- Other Ingredients are: Methyl Parahydroxybenzoate (E 218), Propyl Parahydroxybenzoate (E 216), Povidone, Diethylene Glycol Monoethyl Ether, Glycereth-26 (a Compound of Glycerin and Ethylene Oxide), Hydrochloric Acid and/or Sodium Hydroxide, and Purified Water.
What Cetraxal Plus Looks like and what the Packaging Contains
Cetraxal Plus is a Clear, Colorless or Yellowish Solution for Ear Drops. The Medication Packaging is a White Polyethylene Bottle with a Polyethylene Dropper, Closed with a Polyethylene Cap, in a Cardboard Box.
Each Bottle Contains 10 ml of Solution.
Medical Advice and Education
Antibiotics are Used to Treat Bacterial Infections. They are not Effective against Viral Infections.
If a Doctor has Prescribed Antibiotics, their Use is Necessary due to the Specific Disease Currently Affecting the Patient.
Despite the Use of Antibiotics, some Bacteria may Survive or Multiply. This Phenomenon is Called Resistance: some Antibiotic Therapies Become Ineffective.
Overuse of Antibiotics Leads to Increased Resistance. The Patient may even Contribute to the Development of Resistance in Bacteria and thus Delay Recovery or Reduce the Effectiveness of the Antibiotic if they do not Adhere to:
- Dosage,
- Treatment Schedule,
- Duration of Treatment.
Therefore, to Ensure Effective Treatment, You Should:
- 1. Use Antibiotics only when Prescribed.
- 2. Strictly Follow the Doctor's Recommendations.
- 3. Do not Use Antibiotics Again without a Doctor's Prescription, even if a Similar Disease Occurs.
- 4. Never Give your Antibiotic to Another Person; it may not be Suitable for their Disease
- 5. After Completing Treatment, all Unused Medications Should be Returned to the Pharmacy for Proper Disposal.
For more Detailed Information, the Marketing Authorization Holder or Parallel Importer Should be Contacted.
Marketing Authorization Holder in Spain, the Country of Export:
Laboratorios Salvat, S.A.
C/Gall, 30-36
08950 Esplugues de Llobregat
Barcelona, Spain
Manufacturer:
Pharmaloop, S.L.
C/Bolivia, 15 – Polig. Industrial Azque
28806 Alcalá de Henares
Madrid, Spain
Parallel Importer:
Allpharm Sp. z o.o. sp.k.
ul. M. Zdziechowskiego 11/4
02-659 Warsaw
Repackaged by:
CEFEA Sp. z o.o. Sp.
komandytowa
ul. Działkowa 56
02-234 Warsaw
Synoptis Industrial Sp. z o.o.
ul. Forteczna 35-37
87-100 Toruń
Shiraz Productions Sp. z o.o.
ul. Tymiankowa 24/28
95-054 Ksawerów
Authorization Number in Spain, the Country of Export:866103.9
Parallel Import Authorization Number: 392/24
This Medicinal Product is Authorized for Marketing in the Member States of the European Economic Area under the Following Names:
Spain
Cetraxal Plus 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
France
CETRAXAL 3mg / 0,25mg per ml, Solution for Ear Instillation
Poland
Cetraxal Plus
Czech Republic
Infalin duo 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Denmark
Cetraxal Comp 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Finland
Cetraxal Comp 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Romania
Cexidal 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Slovakia
Infalin duo 3 mg/ml + 0,25 mg/ml Ear Drop Solution
Sweden
Cetraxal Comp 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Norway
Cetraxal Comp
Iceland
Cetraxal Comp 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Germany
InfectoCiproCort 3 mg/ml + 0,25 mg/ml Ear Drops, Solution
Date of Approval of the Leaflet: 13.11.2024
[Information about the Trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Laboratorios Salvat, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Cetraxal PlusDosage form: Drops, (3 mg + 0.25 mg)/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Infectopharm Arzneimittel und Consilium GmbH Laboratorios Salvat, S.A. Pharmaloop S.L.Prescription not requiredDosage form: Drops, (3 mg + 0.25 mg)/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat, S.A.Prescription not requiredDosage form: Drops, 3 mg/ml + 0.25 mg/mlActive substance: fluocinolone acetonide and antiinfectivesPrescription required
Alternatives to Cetraxal Plus in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cetraxal Plus in Ukraine
Alternative to Cetraxal Plus in Spain
Online doctors for Cetraxal Plus
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cetraxal Plus – subject to medical assessment and local rules.






