
OTIX OTIC SOLUTION DROPS

How to use OTIX OTIC SOLUTION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Otix Ear Drops in Solution
Dexamethasone Sodium Phosphate/Polymyxin B Sulfate/Trimethoprim
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What Otix is and what it is used for
- What you need to know before you use Otix
- How to use Otix
- Possible side effects
- Storage of Otix
- Contents of the pack and other information
1. What Otix is and what it is used for
Otix belongs to the group of medicines called dexamethasone and anti-infective combinations. It contains a glucocorticosteroid, dexamethasone phosphate (sodium) and two antibiotics, polymyxin B sulfate and trimethoprim.
The Trimethoprim-Polymyxin B combination has a spectrum of activity that includes gram-positive and gram-negative germs that can grow in the outer ear. The corticosteroid-antimicrobial combination produces a reduction in inflammation of the external auditory canal while combating the existing infection or preventing superinfection in inflammatory processes.
Antibiotics are used to treat bacterial infections and are not effective against viral infections. It is essential that you follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste. |
Otix is indicated for acute otitis externa caused by germs sensitive to the Trimethoprim-Polymyxin B combination in adults and children over 2 years of age.
2. What you need to know before using Otix
Before prescribing this medicine, your doctor will have examined your eardrums to ensure they are not perforated.
Do not use Otix:
- If you are allergic to the active substances or to any of the other components of this medicine (listed in section 6).
- If you have a perforated or damaged eardrum.
- If you have chronic or acute otitis media (infection or inflammation of the middle ear).
- If you have any type of viral, fungal, or tuberculosis infection of the external ear canal.
- Warnings and precautions. This medicine should only be applied in the ear. It should not be ingested or injected. If, once treatment has started, you experience symptoms of itching (urticaria) or allergy (including rash, itching, or respiratory problems), it is recommended that you interrupt the medication and consult your doctor.
- When applying the medicine, avoid contact between the dropper and the ear and fingers to prevent possible contamination.
- With the use of this medicine, you may become more sensitive to other infections. Consult your doctor if symptoms and signs persist after one week of treatment. Consult your doctor if you experience swelling and weight gain around the trunk and face, as these are usually the first manifestations of a syndrome called Cushing's syndrome. Suppression of adrenal gland function may occur after interrupting intensive or long-term treatment with Otix. Consult your doctor before interrupting treatment on your own. These risks are especially important in children and patients treated with a medicine called ritonavir.
Children
Otix is not recommended for use in children under 2 years of age due to the lack of data in these patients.
Other medicines and Otix
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Since systemic absorption is not expected after otic application, it is very unlikely to interact with systemically used medicines. No interaction studies have been performed.
Concomitant administration of other ear medicines is not recommended. If more than one medicine needs to be administered via this route, it is recommended to administer them separately.
Tell your doctor if you are using ritonavir, as it may cause an increase in the amount of dexamethasone in the blood.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
Your doctor will assess the use of this medicine if you are pregnant.
Breastfeeding
Your doctor will assess the use of this medicine if you are breastfeeding.
Driving and using machines
Given the route of administration and the conditions of use, it is unlikely that this medicine will affect your ability to drive or use machines. No data are available.
This medicine contains 0.1 mg of benzalkonium chloride per vial.
3. How to use Otix
Follow your doctor's instructions for administering the medicine exactly. If in doubt, ask your doctor or pharmacist again.
Otic route (administer in the ear).
The recommended dose is 4 drops 3-4 times a day in the affected ear for a minimum of 7 days. If symptoms persist after this, consult your doctor.
Use in children over 2 years of age
No dose adjustment is necessary in this patient group.
ATTENTION: It is essential that when opening the product, you follow the instructions in the "Preparation of the solution" section. The prepared solution will be used during treatment following the instructions in the "Guidelines for correct administration" section. If you have any doubts about preparing the solution, consult your pharmacist.
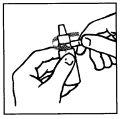
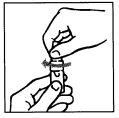
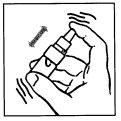
Preparation of the solution.
To prepare the solution, operate with cleanliness and follow the instructions below:
INSTRUCTIONS
|
|
|
|
Guidelines for correct administration.
Once the solution is prepared according to the instructions, unscrew the cap and tilt your head to one side so that the affected ear is facing upwards. Let the drops fall into the affected ear canal with the help of the dropper. Avoid contact between the dropper and the ear and fingers to prevent possible contamination. Keep your head tilted for at least 30 seconds so that the drops penetrate the ear and do not come out. Close the vial after each application and keep it perfectly closed.
If you use more Otix than you should
Due to the characteristics of this preparation, intended for topical use, intoxication phenomena are not expected even if the patient has inadvertently instilled the product without reconstitution.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Telephone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Otix
Apply a single dose as soon as you remember, and continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the missed dose and continue with the next dose of your regular regimen.
Do not use a double dose to make up for missed doses.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
With the use of Otix, the following side effects may appear for which the frequency is not known:
- Growth of non-sensitive microorganisms, particularly fungi, in which case the medication will be discontinued and appropriate measures will be taken.
- Allergic reactions that will require interruption of treatment.
- Local reactions (irritation).
- Ototoxicity.
- Hormonal problems: excessive growth of body hair (especially in women), weakness and muscle wasting, purple striae on the skin of the body, increased blood pressure, irregular or absent menstrual periods, changes in the body's protein and calcium levels, delayed growth in children and adolescents, and swelling and weight gain of the body and face (Cushing's syndrome) (see section 2, "Warnings and precautions").
Corticosteroids can mask the clinical symptoms of infection or sensitization. The side effects typical of corticosteroids are not expected due to the specific conditions of use in the ear.
See also Important information about some of the components of Otix.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Otix
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging, after EXP.
The expiry date is the last day of the month indicated.
Do not store above 30°C. Protect from light.
Once the solution is prepared, it can be stored for 15 days at room temperature.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Otix
- The active substances are dexamethasone (as sodium phosphate), trimethoprim, and polymyxin B (as sulfate).
- The other components are benzalkonium chloride, sulfuric acid, sodium chloride, sodium hydroxide (E 524), sodium citrate tribasic, polysorbate 80, citric acid, and purified water.
Once the solution is prepared: Each ml contains 1 mg of dexamethasone (as sodium phosphate), 1 mg of trimethoprim, and 10,000 IU of polymyxin B (as sulfate).
Appearance of the product and pack contents
A polyethylene bottle with a dropper that contains two solutions (upper and lower) to prepare 5 ml of a clear, colorless solution.
The upper chamber (1 ml) is made of low-density polyethylene and the lower chamber (4 ml) of high-density polyethylene.
Marketing authorisation holder
M4 PHARMA, S.L.
C/ Tánger, 86
08018 Barcelona, Spain
Manufacturer
Laboratori Fundació DAU
c/ C, 12-14 Pol. Ind. Zona Franca
08040 Barcelona – Spain
Date of last revision of this package leaflet:January 2018
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price6.03 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OTIX OTIC SOLUTION DROPSDosage form: OTIC SOLUTION, 0.25 mg / 3.49 mgActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 3 mg/ml + 0.25 mg/mlActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 3mg ciprofloxacin HCl; 0.25mg fluocinolone acetonideActive substance: fluocinolone acetonide and antiinfectivesManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for OTIX OTIC SOLUTION DROPS
Discuss questions about OTIX OTIC SOLUTION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








