How to use Canesten
Leaflet attached to the packaging: patient information
Canesten, 500 mg, vaginal tablet
Clotrimazole
Please read carefully the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
- If after 7 days there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Canesten and what is it used for
- 2. Important information before using Canesten
- 3. How to use Canesten
- 4. Possible side effects
- 5. How to store Canesten
- 6. Contents of the packaging and other information
1. What is Canesten and what is it used for
The active substance of Canesten is clotrimazole, which belongs to a group of medicines called imidazoles. It has a broad spectrum of antifungal activity, meaning it destroys fungi and inhibits their growth. It is effective against microorganisms such as dermatophytes, yeasts, and molds. Canesten is used to treat vaginal and external genital infections caused by microorganisms, such as fungi (usually Candida), that are sensitive to clotrimazole. Symptoms include itching, burning, discharge (thick, white to yellow, odorless vaginal discharge similar to cottage cheese), redness, swelling, and pain. A reliable diagnosis of fungal infection and confirmation of the microorganism's sensitivity to clotrimazole can only be made by a doctor. The information in section 2 of the leaflet should be read to determine when to consult a doctor before using Canesten. The medicine is intended for use in adults and adolescents from 16 years of age.
2. Important information before using Canesten
When not to use Canesten:
- if the patient is allergic to clotrimazole or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Canesten, the patient should discuss it with their doctor:
- if the vaginal infection occurs for the first time,
- if the vaginal infection occurs during the first trimester of pregnancy,
- in case of recurrent infections, if there have been at least 2 episodes of infection in the last 6 months,
- in case of fever over 38°C,
- if the patient experiences lower abdominal pain, back pain,
- in case of foul-smelling discharge,
- if the patient experiences nausea,
- if there is vaginal bleeding and/or pain in the arms.
Canesten should not be used during menstruation. The use of this medicine should be stopped before the start of menstrual bleeding. During the use of Canesten, tampons, vaginal douching, spermicides, or other vaginal products should not be used. During the use of Canesten and during the vaginal infection, sexual intercourse should be avoided, as the infection can be transmitted to the sexual partner. The sexual partner should also be treated locally if they have symptoms of a yeast infection. Treating sexual partners can help prevent reinfection. The contraceptive effectiveness of latex contraceptives, such as condoms and diaphragms, may be reduced. Canesten should not be swallowed.
Children and adolescents
Canesten should not be used in girls under 16 years of age without consulting a doctor.
Canesten and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. The patient should inform their doctor if they are taking tacrolimus or sirolimus, medicines used to control the immune response after organ transplantation. It is possible that the levels of some medicines in the patient's blood may increase if they are used at the same time as Canesten. However, this is unlikely in the case of a single 500 mg dose. If there are any doubts about using another medicine with Canesten, the patient should consult their doctor.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Pregnancy
During the first 3 months of pregnancy, the patient should consult their doctor before using clotrimazole. If Canesten is used during pregnancy, the applicator provided with the packaging should not be used. The vaginal tablet should be inserted into the vagina using the finger to avoid injuring the cervix.
Breastfeeding
Canesten can be used during breastfeeding. If there are any doubts, the patient should consult their doctor or pharmacist.
Driving and using machines
Canesten does not affect the ability to drive or use machines.
3. How to use Canesten
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. If there are any doubts, the patient should consult their doctor or pharmacist. The recommended dose is one vaginal tablet, inserted deeply into the vagina, in a single dose, in the evening.
- one vaginal tablet, inserted deeply into the vagina, in a single dose, in the evening.
The patient should ensure a moist environment in the vagina so that the tablet dissolves completely. Otherwise, undissolved parts of the tablet may slip out of the vagina. The treatment may be repeated. However, recurrent infections may indicate another disease causing the symptoms. If the symptoms recur, the patient should consult their doctor.
Instructions for use
The vaginal tablet should be inserted into the vagina as deeply as possible, using the applicator provided with the medicine, preferably in a lying position, in the evening before sleep. The patient should wash their hands before removing the vaginal tablet and applicator from the packaging and again after using the applicator.
- 1. Remove the applicator from the packaging and pull out the plunger and red lock.
- 2. Place the vaginal tablet in the applicator, with the curved part inside the applicator. Push the plunger and lock into the applicator until a click is felt.
- 3. After feeling the click, remove the lock from the plunger and then carefully insert the applicator into the vagina until the textured area on the handle is reached.
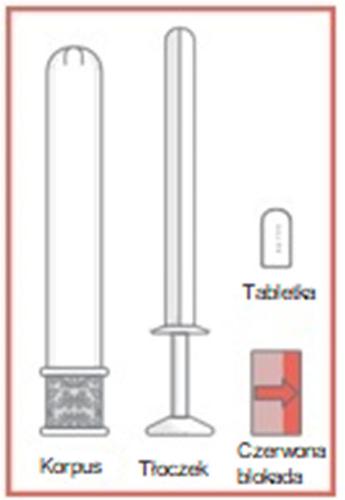
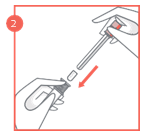
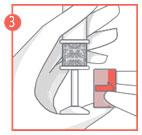
- 4. Hold the applicator at the textured area on the handle and gently push the plunger to the end, until it stops, to apply the vaginal tablet.
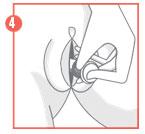
After use, the applicator should be disposed of in a safe place, out of the reach of children. The applicator should not be flushed down the toilet. The method of using vaginal tablets without an applicator is as follows: if the patient is pregnant, the vaginal tablet should be inserted into the vagina using the finger.
Duration of treatment
Canesten is used for the treatment of vaginal infections in a single dose. If after 7 days there is no significant improvement, the patient should consult their doctor to confirm the cause of the infection. If the symptoms worsen, the patient should contact their doctor.
Use in children and adolescents
Canesten should not be used in girls under 16 years of age without consulting a doctor. If there are any further doubts about using this medicine, the patient should consult their doctor.
4. Possible side effects
Like all medicines, Canesten can cause side effects, although not everybody gets them. Side effects have been observed with the following frequency:
Common, more than 1 in 100 people:
- burning
Uncommon, more than 1 in 1000 people:
- abdominal pain
- itching
- redness of the skin and/or irritation
Rare, more than 1 in 10,000 people:
- allergic reactions
- swelling
- skin rash
- vaginal bleeding
Frequency not known, based on available data:
- vaginal erosion
- feeling of discomfort in the vagina
- vaginal pain
- nausea
- hives
- discharge
- pain
If the patient experiences any side effects, they should stop using the medicine and inform their doctor or pharmacist. Local side effects may be similar to the symptoms of the disease, making it difficult to distinguish between the symptoms of the infection and the side effects caused by the medicine.
Reporting side effects
If the patient experiences any side effects, including those not listed in the leaflet, they should inform their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Canesten
The medicine should be stored out of sight and reach of children. Do not store above 25°C. Do not use this medicine after the expiry date stated on the carton and blister after: EXP. The expiry date refers to the last day of the month. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Canesten contains
- The active substance is clotrimazole.
- The other ingredients (excipients) are: calcium lactate pentahydrate; microcrystalline cellulose; hypromellose 15cP; lactic acid; lactose monohydrate; corn starch; magnesium stearate; colloidal silicon dioxide, anhydrous; crospovidone.
What Canesten looks like and contents of the pack
White vaginal tablet with markings MU and Bayer. One vaginal tablet, in a blister and applicator, in a cardboard box.
Marketing authorization holder
Bayer Sp. z o.o. Al. Jerozolimskie 158 02-326 Warsaw tel. +48 22 5723500
Manufacturer
Bayer AG Kaiser-Wilhelm Allee D-51368 Leverkusen Germany GP Grenzach Produktions GmbH Emil-Barell-Strasse 7 79639 Grenzach-Wyhlen Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Finland: Canesten, 500 mg vaginal tablet Poland: Canesten, 500 mg, vaginal tablet Date of last revision of the leaflet:05.2022
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterGP Grenzach Produktions GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CanestenDosage form: Tablets, 500 mgActive substance: clotrimazoleManufacturer: GP Grenzach Produktions GmbHPrescription not requiredDosage form: Tablets, 500 mgActive substance: clotrimazolePrescription not requiredDosage form: Cream, 10 mg/gActive substance: clotrimazoleManufacturer: Laboratorios Basi - Industria Farmaceutica, S.A.Prescription not required
Alternatives to Canesten in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Canesten in Ucrania
Alternative to Canesten in España
Online doctors for Canesten
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Canesten – subject to medical assessment and local rules.