

ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER

Ask a doctor about a prescription for ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER

How to use ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER
Introduction
PACKAGE LEAFLET
Package Leaflet:Information for the patient
Elymbus 0.1 mg/g eye gel in single-dose container
bimatoprost
Read the entire package leaflet carefully before starting to use this medicine,as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Elymbus and what is it used for
- What you need to know before using Elymbus
- How to use Elymbus
- Possible side effects
5 Conservation of Elymbus
- Contents of the container and additional information
1. What is Elymbus and what is it used for
Elymbus is a medicine for glaucoma. Elymbus belongs to a group of medicines called prostamides.
Elymbus eye gel is used to reduce high pressure in the eye in adults. This medicine can be used alone or with other eye drops called beta-blockers that also reduce pressure.
The eye contains a clear, watery liquid that keeps the inside of the eye healthy. This liquid is constantly drained out of the eye and new liquid is produced to replace it. If the liquid does not drain quickly enough, the pressure inside the eye increases. This medicine works by increasing the drainage of the liquid. This reduces the pressure inside the eye. If this pressure is not reduced, it could lead to a disease called glaucoma and damage your vision.
2. What you need to know before using Elymbus
Do not use Elymbus:
- If you are allergic to bimatoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Elymbus.
Consult your doctor if:
- you have any respiratory problems.
- you have liver or kidney problems.
- you have had cataract surgery in the past.
- you have low blood pressure or a slow heart rate.
- you have had a viral infection or inflammation of the eye.
During treatment, Elymbus may cause fat loss around the eye, which can lead to deepening of the eyelid fold, sunken eyes (enophthalmos), drooping of the upper eyelid (ptosis), stretching of the skin around the eye (involution of the dermatocalasis), and the white part of the eye becoming more visible (inferior scleral exposure). The changes are usually mild, but if they worsen, they can affect your field of vision. The changes may disappear if you stop using Elymbus. Elymbus may also cause darkening and growth of the eyelashes, as well as darkening of the skin around the eyelid. The color of the iris may darken. These changes may be permanent and more visible if only one eye is being treated.
Children and adolescents
Elymbus has not been studied in children under 18 years of age and should not be used in patients under 18 years of age.
Other medicines and Elymbus
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Elymbus may pass into breast milk, so you should not use it if you are breastfeeding.
Driving and using machines
After applying Elymbus, you may experience blurred vision for a short time. Do not drive or use machines until you can see clearly.
3. How to use Elymbus
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
Elymbus should only be used in the eye. The recommended dose is one drop in each eye that needs treatment, once a day, at night.
If you use Elymbus with another eye medication, you should administer it at least 15 minutes before Elymbus. Elymbus should be used last.
Do not use the medicine more than once a day, as this may reduce the effectiveness of the treatment.
Users of contact lenses
If you use contact lenses, you should remove them before using Elymbus. After applying Elymbus, you should wait 15 minutes before putting your contact lenses back in.
Instructions for use
This medicine should be administered in the eye.
Follow these instructions to use the eye drops:
- Wash your hands and sit or stand comfortably.
- Open the pouch containing 10 single-dose containers.
- Separate one single-dose container from the strip.
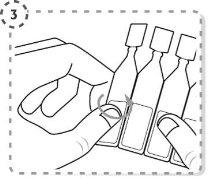
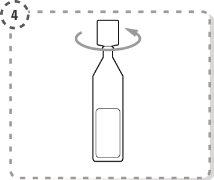
- Twist the tip of the single-dose container as shown. Do not touch the tip after opening the container.
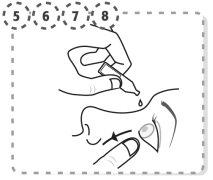
- Use your finger to gently separate the lower eyelid of the affected eye.
- Tilt your head back and look up at the ceiling.
- Place the tip of the single-dose container close to the eye, without touching it.
- Gently press the single-dose container so that one drop falls into the eye, then remove your finger from the lower eyelid.
- Repeat the operation in the other eye, if your doctor has indicated it. Each single-dose container contains enough for both eyes.
- Release the lower eyelid and close your eyes for a moment.
- Wipe away any excess that runs down your cheek. If a drop does not reach your eye, try again.
- Discard the single-dose container after use. Do not save it for later use. As the sterility of the single-dose container cannot be guaranteed after opening (preservative-free eye gel), a new single-dose container should be opened before each use.
- Wipe away any excess liquid with a clean cloth.
- Place the unused single-dose containers inside the pouch. Unused single-dose containers should be used within 1 month of opening the pouch.
If you use more Elymbus than you should
If you have applied more drops in the eye than you should, it is unlikely to cause you any serious harm. Apply the next dose at the usual time. If you are concerned, talk to your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Elymbus
If you forget to apply Elymbus, use one drop as soon as you remember and then return to your usual routine. Do not apply a double dose to make up for the forgotten dose.
If you stop using Elymbus
Elymbus should be used every day for it to work well. If you stop using Elymbus, the pressure inside the eye may increase, so consult your doctor before stopping treatment.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur with Elymbus:
Common side effects
May affect up to 1 in 10 people
Affecting the eye
- Mild redness
- Eye pain
- Eye irritation
- Redness and eye discomfort
- Sensation of having something in the eye
- Dry eye
- Itchy eye
- Temporary difficulty seeing clearly
Uncommon side effects
May affect up to 1 in 100 people
Affecting the eye
- Small epithelial erosions in the eye, with or without inflammation
- Sensations such as numbness, tingling, and pinching in the eye
- Inflamed eyelids
- Loss of eyelashes
- Longer eyelashes
- Sensitivity to light
- Increased tearing
- Darker eyelashes
- Darker eyelid color
- Swollen eyelid
- Dry, red, and itchy eyelid skin
Affecting the body
- Dizziness
The following side effects have been observed with bimatoprost eye drops in solution (0.1 mg/ml, formulation with preservatives):
Very common side effects
May affect more than 1 in 10 people
Affecting the eye
- Mild redness (up to 29% of people)
- Fat loss in the eye area that can cause deepening of the eyelid fold, sunken eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of the dermatocalasis), and the white part of the eye becoming more visible (inferior scleral exposure).
Common side effects
May affect up to 1 in 10 people
Affecting the eye
- Small epithelial erosions in the eye, with or without inflammation
- Irritation
- Itchy eyes
- Longer eyelashes
- Irritation when instilling the drop in the eye
- Eye pain
Affecting the skin
- Red and itchy eyelids
- Darker skin color around the eye.
- Hair growth around the eye.
Uncommon side effects
May affect up to 1 in 100 people
Affecting the eye
- Darker iris color
- Eye fatigue
- Inflammation of the eye surface
- Blurred vision
- Loss of eyelashes
Affecting the skin
- Dry skin
- Flaking of the eyelid margin
- Swollen eyelid
- Itching
Affecting the body
- Headache
- Feeling unwell
Side effects of unknown frequency
Affecting the eye
- Macular edema (inflammation of the retina in the back of the eye that can lead to worsening of vision)
- Darker eyelid
- Dryness
- Sticky eyes
- Sensation of a foreign body in the eye
- Eye inflammation
- Increased tearing
- Eye discomfort
- Sensitivity to light
Affecting the body
- Asthma
- Difficulty breathing
- Symptoms of allergic reaction (inflammation, redness of the eye, and skin rash)
- Dizziness
- High blood pressure
- Discoloration of the skin (periocular)
In addition to the side effects described above, the following side effects have been observed with another medicine that contains a higher concentration of bimatoprost (0.3 mg/ml):
- Eye burning
- An allergic reaction in the eye
- Inflamed eyelids
- Difficulty seeing clearly
- Inflammation of the clear layer that covers the eye
- Tearing
- Darker eyelashes
- Retinal hemorrhage
- Inflammation inside the eye
- Cystoid macular edema (inflammation of the retina in the back of the eye that leads to worsening of vision)
- Eyelid twitching
- The eyelid has contracted away from the eye surface
- Redness of the skin around the eye
- Weakness
- An increase in blood test results that show liver activity
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Conservation of Elymbus
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box, pouch, and single-dose containers after EXP. The expiration date is the last day of the month indicated.
This medicine does not require special temperature conditions for storage.
After the first opening of the pouch:use the single-dose containers within 1 month.
To help you remember, write the date of first opening of the pouch.
Keep the unused single-dose containers inside the opened pouch to protect them from light.
After the first opening of the single-dose container:use immediately and discard the single-dose container after use.
Medicines should not be disposed of through wastewater or household waste. Deposit the containers and medicines you no longer need at the SIGRE collection point in your pharmacy. If you have any doubts, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the container and additional information
Composition of Elymbus
The active ingredient is bimatoprost. Each gram of gel contains 0.1 mg of bimatoprost.
The other ingredients are: sorbitol, carbomer, sodium acetate trihydrate, macrogol, sodium hydroxide (pH adjustment), water for injectable preparations.
Appearance of the product and contents of the container
Elymbus is a colorless, opalescent eye gel in single-dose containers. It is presented in single-dose containers inside a pouch with 10 units.
Each single-dose container contains 0.3 g of product.
The boxes contain 10, 30 (3 pouches with 10), or 90 (9 pouches with 10) single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Laboratoires THEA
12, rue Louis Blériot
63100 CLERMONT-FERRAND
France
Manufacturer
LABORATOIRE UNITHER
1 rue de l’Arquerie
50200 Coutances
France
You can request more information about this medicine from the local representative of the marketing authorization holder:
LABORATORIOS THEA, S.A.
C/ Enric Granados nº 86-88, 2ª planta
08008 Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Croatia, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, Greece, Netherlands, Hungary, Ireland, Iceland, Italy, Latvia, Lithuania, Luxembourg, Norway, Poland, Portugal, Czech Republic, Romania, Sweden ……………………………………………………………… Elymbus
France ……………………………………………………………………………… Elumibus
Date of the last revision of this package leaflet: May 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINERDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Sifi S.P.A.Prescription requiredDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Laboratorio Stada S.L.Prescription required
Alternatives to ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER in Poland
Alternative to ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER in Ukraine
Online doctors for ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for ELYMBUS 0.1 mg/g OPHTHALMIC GEL SINGLE-DOSE CONTAINER – subject to medical assessment and local rules.














