
Befoair
Ask a doctor about a prescription for Befoair

How to use Befoair
Leaflet accompanying the packaging: information for the user
Befoair, (200 micrograms + 6 micrograms)/metered dose, inhalation aerosol, solution
Beclometasone dipropionate + Formoterol fumarate dihydrate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Befoair and what is it used for
- 2. Important information before using Befoair
- 3. How to use Befoair
- 4. Possible side effects
- 5. How to store Befoair
- 6. Package contents and other information
1. What is Befoair and what is it used for
Befoair is a solution in an inhalation aerosol, containing two active substances, which are delivered directly to the lungs when you inhale through the mouthpiece of the inhaler. The medicine contains two active substances: Beclometasone dipropionate, which belongs to a group of medicines called corticosteroids, which have an anti-inflammatory effect by reducing swelling and irritation of the lungs. Formoterol fumarate dihydrate, which belongs to a group of long-acting bronchodilators, which relax the muscles in the airways and make breathing easier. Together, these two active substances make breathing easier. They also help prevent asthma symptoms, such as shortness of breath, wheezing, and coughing. Asthma Befoair is intended for regular treatment of asthma in adults, in whom:
- asthma symptoms are not adequately controlled with inhaled corticosteroids and a short-acting bronchodilator used as needed, or
- adequate control of asthma symptoms has been achieved with both inhaled corticosteroids and long-acting bronchodilators.
2. Important information before using Befoair
When not to use Befoair:
- if the patient is allergic to beclometasone dipropionate or formoterol fumarate dihydrate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Befoair, the patient should discuss with their doctor, pharmacist, or nurse if they have any of the following conditions:
- heart disease, such as angina pectoris (chest pain), recent myocardial infarction (heart attack), heart failure, coronary heart disease, heart valve defects, or any other heart disease, or a disease known as hypertrophic obstructive cardiomyopathy (HOCM), which is a condition where the heart muscle is abnormal
- arterial narrowing (also known as atherosclerosis), if the patient has high blood pressure or a diagnosed aneurysm (abnormal dilation of a blood vessel)
- heart rhythm disorders (rapid or irregular heartbeat), rapid heart rate, or palpitations, or if there is any information about abnormal heart rhythm
- hyperthyroidism
- low potassium levels in the blood
- any liver or kidney disease
- diabetes (inhalation of high doses of formoterol may increase blood glucose levels, so before starting treatment with this medicine, as well as from time to time during treatment, additional blood tests may be necessary to determine blood sugar levels)
- adrenal gland tumor (pheochromocytoma)
- the patient is scheduled for general anesthesia. Depending on the type of anesthesia, it may be necessary to discontinue Befoair for at least 12 hours before anesthesia.
- if the patient is being treated or has been treated for tuberculosis or if the patient has a viral or fungal infection in the chest
- if the patient needs to avoid consuming alcohol for any reason.
Before using Befoair, the patient should always inform their doctor if any of the above warnings apply to them.
Before using this medicine, the patient should consult a doctor, nurse, or pharmacist if they have any current or past health problems or allergies, or if they are unsure whether Befoair can be used.
The doctor may occasionally order a blood test to check potassium levels, especially
in patients with severe asthma.Like other bronchodilators, Befoair may cause a sudden decrease in potassium levels in the blood (hypokalemia). This is related to low oxygen levels in the blood caused by taking other medicines at the same time as Befoair, which can exacerbate the decrease in potassium levels in the blood.
If the patient is taking high doses of inhaled corticosteroids for a long time, they may be more likely to require corticosteroid administration in stressful situations. Stressful situations may include hospitalization after an accident, severe injury, or pending surgery. In such situations, the doctor will decide whether to increase the corticosteroid dose or prescribe other steroids in the form of tablets or injections.
If hospitalization is necessary, the patient should remember to bring all their medicines and inhalers, including Befoair, as well as over-the-counter medicines, if possible, in their original packaging.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Children and adolescents
Befoair should not be used in children and adolescents under the age of 18.
Befoair and other medicines
The patient should tell their doctor, pharmacist, or nurse about all the medicines they are taking, including those purchased without a prescription. This is necessary because Befoair may affect the action of some other medicines. Similarly, some medicines may affect the action of Befoair.
Concomitant use of Befoair with:
- HIV medicines ritonavir and cobicistat, as well as some other medicines, may enhance the effect of Befoair, and the doctor may want to closely monitor the patient's condition when taking such medicines
- beta-blocker medicines, including eye drops, may reduce the effect of formoterol or formoterol may not work at all. Beta-blocker medicines are used to treat many diseases, including heart disease, high blood pressure, and glaucoma (increased pressure in the eye).
- beta-agonist medicines (medicines that work in the same way as formoterol) - may enhance the effect of formoterol
- medicines used to treat irregular heart rhythm (quinidine, disopyramide, procainamide), medicines used to treat allergies (antihistamines), or medicines used to treat symptoms of depression or mental disorders, such as monoamine oxidase inhibitors (e.g., phenelzine and isocarboxazid), tricyclic antidepressants (e.g., amitriptyline and imipramine), phenothiazines may cause certain changes in the electrocardiogram (ECG, a record of heart activity). They may also increase the risk of heart rhythm disorders (ventricular arrhythmias).
- medicines used to treat Parkinson's disease (L-dopa), medicines used to treat hypothyroidism (L-thyroxine), medicines containing oxytocin (which cause uterine contractions), or alcohol may reduce the heart's tolerance to beta-agonists, such as formoterol
- medicines used to treat mental disorders, such as monoamine oxidase inhibitors (MAOIs) or medicines with similar properties, such as furazolidone and procarbazine, may cause an increase in blood pressure
- medicines used to treat heart disease (digoxin) may cause a decrease in potassium levels in the blood. This may increase the risk of heart rhythm disorders
- other medicines used to treat asthma (theophylline, aminophylline, or steroids) and diuretic medicines (diuretic tablets) may cause a decrease in potassium levels in the blood.
- certain general anesthetics containing halogenated hydrocarbons (used during operations and dental procedures) may increase the risk of heart rhythm disorders
Pregnancy, breastfeeding, and fertility
There are no clinical data on the use of Befoair during pregnancy.
Befoair should not be used if the patient is pregnant, thinks they may be pregnant, or plans to have a baby, or if the patient is breastfeeding, unless the doctor decides otherwise.
Driving and using machines
It is unlikely that Befoair will affect the ability to drive and use machines.
Befoair contains alcohol
Befoair contains 9 mg of alcohol (ethanol) per actuation, which is equivalent to 0.25 mg/kg body weight per dose, when two actuations are used. The amount of alcohol in two actuations of this medicine is equivalent to less than 1 mL of beer or wine. The small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Befoair
This medicine should always be used as directed by the doctor or pharmacist. If you have any doubts, you should consult a doctor or pharmacist.
The doctor will regularly check that the patient is taking the optimal dose of Befoair.
The doctor will determine the smallest dose that provides the best control of asthma symptoms.
Dosage:
Adults and the elderly:
The recommended dose is two actuations twice daily.
The maximum daily dose is 4 actuations.
Remember: You should always have a fast-acting inhalation medicine with you to relieve asthma symptoms or an acute asthma attack.
Special patient groups:
There is no need to adjust the dose in elderly patients. There is no information on the use of Befoair in patients with liver or kidney function disorders.
Use in children and adolescents under 18 years of age:
This medicine should NOT be used in children and adolescents under 18 years of age.
The dose of beclometasone dipropionate in Befoair, which is effective in treating asthma, may be lower than the dose contained in other inhaled medicines containing beclometasone dipropionate. If the patient has previously used another inhaled medicine containing
beclometasone dipropionate, the doctor will prescribe the appropriate dose of Befoair.
Do not increase the dose
If you feel that the effect of the medicine is insufficient, you should always consult a doctor before increasing the dose of the medicine.
If breathing difficulties worsen:
If shortness of breath or wheezing (breathing with a whistling sound) worsens immediately after inhaling the medicine, you should stop using Befoair immediately and use a fast-acting inhalation medicine to relieve the symptoms. You should contact your doctor as soon as possible. The doctor will assess the symptoms and, if necessary, prescribe other treatment. See also section 4, "Possible side effects".
If asthma symptoms worsen:
If the symptoms of the disease worsen or become more difficult to control (e.g., if you need to use another inhalation medicine to relieve the symptoms more frequently) or if the inhalation medicine does not relieve the symptoms, you should inform your doctor immediately. This may indicate that the asthma is worsening, and the doctor may decide to change the dose of Befoair or prescribe other treatment.
Method of administration:
Befoair is intended for inhalation use
This medicine is in a pressurized container, in a plastic casing with a mouthpiece. At the back of the inhaler, there is a dose counter, which shows how many doses of the medicine are left. After each actuation of the inhaler, a dose of the medicine is released, and the dose counter decreases by one. Be careful not to drop the inhaler, as this may cause the dose counter to decrease by one.
Testing the inhaler
Before using the inhaler for the first time, or if the inhaler has not been used for 14 days or more, you should perform an inhaler test to ensure that it is working properly.
- 1. Remove the protective cap from the mouthpiece.
- 2. Hold the container in an upright position with the mouthpiece pointing downwards.
- 3. Point the mouthpiece away from you and firmly press the top of the container to release one actuation.
- 4. Check the dose counter. If the inhaler is being tested for the first time, the dose counter should show 120.
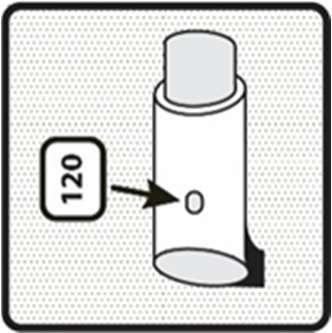
How to use the inhaler
If possible, you should stand or sit upright while inhaling.
Before inhaling, check the dose counter: any number between "1" and "120" indicates that there are still doses of the medicine left in the container. If the dose counter shows "0", it means that there are no more doses of the medicine left, and you should discard the old inhaler and purchase a new one.
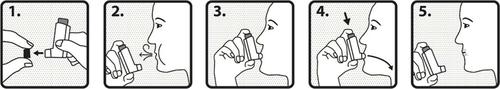
- 1. Remove the protective cap from the mouthpiece and check that the mouthpiece is clean and free of dust or other contaminants.
- 2. Exhale slowly and deeply.
- 3. Hold the container in an upright position, with the stem pointing upwards, and then place the mouthpiece between your lips. Do not bite the mouthpiece.
- 4. Inhale slowly and deeply through your mouth. As soon as you start inhaling, firmly press the top of the inhaler to release one actuation of the medicine.
- 5. Hold your breath for as long as possible, then remove the inhaler from your mouth and exhale slowly. Do not exhale into the inhaler. To take another dose of the medicine, keep the inhaler in an upright position for about half a minute, then repeat the steps from 2 to 5. Important:Do not perform the steps from 2 to 5 too quickly.
After use, replace the protective cap and check the dose counter. To reduce the risk of fungal infections of the mouth and throat, you should rinse your mouth or throat with water after each inhalation, or brush your teeth.
If the dose counter shows 20, you should have a new inhaler ready.
You should stop using the inhaler if the counter shows 0, as the medicine left in the container may not provide a full dose.
If a "mist" appears over the top opening of the mouthpiece or to the side of the mouth, it means that Befoair is not reaching the lungs as it should. You should take another dose, following the instructions, starting again from step 2.
People with a weak grip will find it easier to hold the inhaler with both hands: they should then place both index fingers on the top of the inhaler and both thumbs on the base.
If you feel that the effect of Befoair is too strong or too weak, you should consult a doctor or pharmacist.
If you have difficulty using the inhaler during inhalation, you can use a spacer device (Aerochamber Plus). You should ask your doctor or pharmacist about this device.
It is essential to read the leaflet that comes with the Aerochamber Plus spacer device and follow the instructions for use and cleaning.
Cleaning the inhaler:
The inhaler should be cleaned once a week.
When cleaning, do not remove the container from the plastic casing, and do not use water or other liquids to clean the inhaler.
Cleaning the inhaler:
- 1. Remove the protective cap from the mouthpiece by pulling it straight off the inhaler.
- 2. Wipe the mouthpiece and dose counter with a dry cloth or tissue from the inside and outside.
- 3. Replace the protective cap.
Using more than the recommended dose of Befoair
- using more than the recommended dose of formoterol may cause the following symptoms: nausea, vomiting, rapid heartbeat, palpitations, heart rhythm disorders, changes in the electrocardiogram (ECG), headache, tremors, drowsiness, excessive acidic products in the blood, decreased potassium levels in the blood, increased glucose levels in the blood. The doctor may order a blood test to check potassium and glucose levels.
- using too much beclometasone dipropionate may cause temporary adrenal gland disorders, which resolve on their own within a few days. The doctor may order a blood test to check cortisol levels in the blood. If you experience any of these symptoms, you should inform your doctor.
Missing a dose of Befoair
You should take the missed dose as soon as possible. If it is almost time for the next dose, do not take the missed dose, just take the next dose at the usual planned time. Do not take a double dose to make up for the missed dose.
Stopping treatment with Befoair
Even if you feel better, you should not stop using Befoair or reduce the dose without consulting your doctor. It is essential to regularly use Befoair, even when the symptoms of the disease have resolved.
If you have any further doubts about using this medicine, you should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Befoair can cause side effects, although not everyone gets them.
As with other inhaled medicines, there is a risk of worsening shortness of breath and wheezing immediately after taking Befoair, known as paradoxical bronchospasm.You should immediately STOP taking Befoairand use a fast-acting inhalation bronchodilator to relieve the symptoms of shortness of breath and wheezing. You should contact your doctor immediately.
You should immediately inform your doctorif you experience any allergic reactions, such as skin allergies, itching, skin rash, skin redness, skin swelling, or swelling of the skin and mucous membranes, especially of the eyes, face, lips, and throat.
Other possible side effects are listed below, depending on their frequency.
- fungal infections (of the mouth and throat)
- headache
- hoarseness
- sore throat. Less common (may affect up to 1 in 100 people):
- palpitations, abnormal rapid heartbeat, and heart rhythm disorders
- changes in the electrocardiogram (ECG)
- increased blood pressure
- flu-like symptoms
- sinusitis
- nasal congestion
- ear infection
- throat irritation
- cough and cough with expectoration
- asthma attack
- vaginal fungal infections
- nausea
- taste disturbances
- mouth burning
- dryness of the mouth mucosa
- difficulty swallowing
- indigestion
- stomach upset
- diarrhea
- muscle pain and muscle cramps
- redness of the face and throat
- increased blood flow to some tissues
- increased sweating
- tremors
- restlessness, especially motor restlessness
- dizziness
- hives
- changes in some blood test results:
- decreased white blood cell count
- increased platelet count
- decreased potassium levels in the blood
- increased glucose levels in the blood
- increased insulin, free fatty acids, and ketone bodies in the blood.
Rare (may affect up to 1 in 1,000 people):
- feeling of pressure in the chest
- heart rhythm disorders (caused by premature contraction of the heart chambers)
- decreased blood pressure
- kidney inflammation
- skin and mucous membrane swelling, lasting for several days. Very rare (may affect up to 1 in 10,000 people):
- shortness of breath
- worsening of asthma
- decreased platelet count
- swelling of the hands and feet.
Unknown (frequency cannot be estimated from available data):
- blurred vision These side effects are more likely to occur in children:
- sleep disturbances
- depression or feeling of sadness
- nervousness
- excessive excitement or irritability.
The following side effects have also been reported in patients with chronic obstructive pulmonary disease:
- pneumonia (common): you should inform your doctor if you experience any of the following symptoms: increased production of sputum, change in the color of sputum, fever, increased cough, increased breathing difficulties
- decreased cortisol levels in the blood (uncommon), which is caused by the effect of corticosteroids on the adrenal glands
- irregular heartbeat (uncommon). The use of inhaled corticosteroids in high doses for a long time may rarely cause systemic effects, including:
- adrenal gland disorders (adrenal gland suppression)
- decreased bone density (osteoporosis)
- delayed growth in children and adolescents
- increased intraocular pressure (glaucoma)
- cataract.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw,
phone: (22) 49 21 301, fax: (22) 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Befoair
The medicine should be stored out of sight and reach of children.
Information for the pharmacist:
Store in a refrigerator (2°C to 8°C) for a maximum of 18 months.
Information for the patient:
Do not use the medicine after 3 months from the date of dispensing from the pharmacy and never use this medicine after the expiry date stated on the carton and label after: "Expiry date (EXP)".
The expiry date refers to the last day of the month.
Store below 25°C, for a maximum of 3 months.
If the inhaler has been exposed to very low temperatures, you should warm it in your hands for a few minutes before use. Never use other methods to heat the container.
Warning: The container contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not pierce the container.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Befoair contains
- The active substances of the medicine are beclometasone dipropionate and formoterol fumarate dihydrate. One metered dose from the inhaler contains 200 micrograms of beclometasone dipropionate and 6 micrograms of formoterol fumarate dihydrate. This corresponds to a delivered dose through the mouthpiece, containing 177.7 micrograms of beclometasone dipropionate and 5.1 micrograms of formoterol fumarate dihydrate.
- This medicine contains fluorinated greenhouse gases. Each inhaler contains 10.24 g of HFA-134a, which corresponds to 0.015 tons of CO2 equivalent (GWP = 1430).
- The other ingredients are norflurane (HFA 134a), anhydrous ethanol, concentrated hydrochloric acid (to adjust pH).
What Befoair looks like and what the package contains
Befoair is available as a solution for inhalation, in an aluminum container with a metering valve, placed in a plastic casing with a dose counter and a plastic protective cap.
Each cardboard box contains:
1 container (which delivers 120 doses).
Marketing authorization holder
Orion Corporation
Orionintie 1
02200 Espoo
Finland
Importer
Orion Corporation Orion Pharma
Orionintie 1
02200 Espoo
Finland
To obtain more detailed information about this medicine, you should contact the local representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o. o.
[email protected]
Date of last revision of the leaflet:2024-12
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterOrion Corporation Orion Pharma
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BefoairDosage form: Aerosol, (100 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription requiredDosage form: Aerosol, (200 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription requiredDosage form: Aerosol, (100 mcg + 6 mcg)/measured doseActive substance: formoterol and beclometasonePrescription not required
Alternatives to Befoair in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Befoair in Spain
Alternative to Befoair in Ukraine
Online doctors for Befoair
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Befoair – subject to medical assessment and local rules.














