How to use Azelastin Comod
PATIENT INFORMATION LEAFLET
Azelastin COMOD,
0.5 mg/ml, eye drops, solution
Azelastini hydrochloridum
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- This medicine should always be used exactly as described in the patient information leaflet or as directed by a doctor or pharmacist. Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 2 days there is no improvement or you feel worse, contact your doctor.
Table of contents of the leaflet:
- 1. What is Azelastin COMOD and what is it used for
- 2. Important information before using Azelastin COMOD
- 3. How to use Azelastin COMOD
- 4. Possible side effects
- 5. How to store Azelastin COMOD
- 6. Contents of the pack and other information
1. What is Azelastin COMOD and what is it used for
Azelastin COMOD contains the active ingredient azelastine hydrochloride, which belongs to the group of antihistamine medicines. The action of antihistamines is to prevent the action of substances such as histamine, which is produced by the body during an allergic reaction. Azelastine has been shown to relieve eye inflammation. Azelastin COMOD can be used to treat and prevent eye disorders caused by hay fever (seasonal allergic conjunctivitis) in adults and children aged 4 years and older. Azelastin COMOD can also be used to treat eye disorders caused by allergies to substances such as house dust mites or animal hair (perennial allergic conjunctivitis) in adults and children aged 12 years and older.
Azelastin COMOD is not suitable for the treatment of eye infections.
Edition 07/2024
- 1.3.1 SPC, labelling and package leaflet
2. Important information before using Azelastin COMOD
When not to use Azelastin COMOD:
- if you are allergic to azelastine hydrochloride or any of the other ingredients of Azelastin COMOD (see section 6 "What Azelastin COMOD contains").
Warnings and precautions
Before using Azelastin COMOD, discuss it with your doctor or pharmacist
- if you are not sure whether your eye symptoms are caused by an allergy. In particular, if the symptoms affect only one eye, there is blurred vision or eye pain, and you do not have nasal symptoms, it is more likely to be an eye infection rather than an allergy.
- if the symptoms worsen or persist for more than 48 hours without significant improvement despite using Azelastin COMOD
- If you wear contact lenses.
Children and adolescents
In the treatment of eye disorders caused by hay fever (seasonal allergic conjunctivitis):
The medicine should not be used in children under 4 years of age because the safety and efficacy have not been established.
In the treatment of eye disorders caused by perennial allergic conjunctivitis:
The medicine should not be used in children under 12 years of age because the safety and efficacy have not been established.
Azelastin COMOD and other medicines
No interactions with other medicines have been reported. Tell your doctor about all the medicines you have taken recently and are taking, and the medicines you plan to take.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Temporary blurred vision may occur after using Azelastin COMOD.
In this case, wait until the symptoms have resolved before driving or operating machinery.
3. How to use Azelastin COMOD
This medicine should always be used exactly as directed by your doctor or pharmacist.
If you are unsure, consult your doctor or pharmacist.
Remember:
Edition 07/2024
- 1.3.1 SPC, labelling and package leaflet
- Azelastin COMOD should only be used for the eyes.
The usual dose of the medicine, unless your doctor advises otherwise:
Eye disorders caused by hay fever (seasonal allergic conjunctivitis)
- In adults and children aged 4 years and older
- usually, 1 drop is instilled into each eye in the morning and evening.
In case of expected exposure to the allergen, the dose of Azelastin COMOD should be used prophylactically before leaving the house.
Allergic eye disorders (perennial allergic conjunctivitis)
- In adults and children aged 12 years and older
- usually, 1 drop is instilled into each eye in the morning and evening.
In case of severe symptoms, your doctor may increase the dose to 1 drop into each eye, four times a day.
The allergic eye symptoms should resolve within 15-30 minutes.
Method of administration
Wash your hands.
Using a clean, single-use tissue, gently wipe the lower eyelid margin.
Fig. 1:
Before each use, remove the cap from the dropper.
Before the first use of Azelastin COMOD, point the bottle with the dropper vertically downwards and press the bottom of the bottle several times until the first drop of solution appears. After performing this action, the bottle is ready to administer the drops.
Fig. 2:
Holding the bottle with the dropper downwards, place your thumb on the top of the bottle and your other fingers on the bottom.
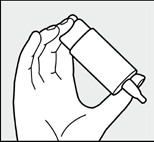
Fig. 3:
Rest the hand holding the Azelastin COMOD bottle on the other hand, as shown in the figure.
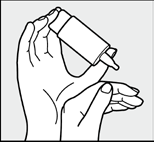
Fig. 4:
Tilt your head back slightly, use your free hand to gently pull down the lower eyelid and quickly and firmly press the center of the bottle. This will activate the mechanism to deliver one drop of solution. Thanks to the special valve technology of the COMOD system, the size of the drop and the speed of its delivery will always be the same, regardless of the pressure on the bottom. After instillation, slowly close your eyes, allowing the solution to spread evenly over the surface of your eye.
Edition 07/2024
- 1.3.1 SPC, labelling and package leaflet
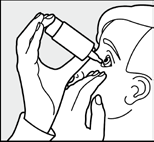
 | of the eye. |
| Fig. 5: Then release the eyelid, and then gently press the inner corner of the eye from the side of the nose (Fig. 5), perform a few gentle blinks to distribute the medicine evenly over the surface of the eye. | |
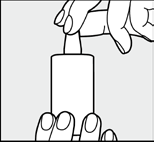 | Fig. 6: After each use, immediately close the bottle tightly with the cap. Make sure the tip of the dropper is dry. |
Duration of treatment
If possible, Azelastin COMOD should be used regularly until the symptoms resolve.
Do not use Azelastin COMOD for more than 6 weeks.
Using more than the recommended dose of Azelastin COMOD
In case of accidental administration of too much Azelastin COMOD into the eye, no problems should occur.
If you are concerned, contact your doctor.
In case of accidental ingestion of Azelastin COMOD, immediately contact your doctor or the nearest hospital emergency department.
Missing a dose of Azelastin COMOD
Instill the eye drops as soon as possible and then continue with the treatment at the usual time. Do not use a double dose to make up for the missed dose.
Stopping the use of Azelastin COMOD
If you stop using Azelastin COMOD, the symptoms of the disease will likely return.
If you have any further doubts about the use of the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Azelastin COMOD can cause side effects, although not everybody gets them.
These side effects are:
Common (may affect up to 1 in 10 people):
mild eye irritation (burning, itching, tearing) after administration of Azelastin COMOD. These symptoms should resolve quickly.
Uncommon (may affect up to 1 in 100 people):
bitter taste in the mouth. It should resolve quickly, especially after taking a non-alcoholic drink.
Edition 07/2024
- 1.3.1 SPC, labelling and package leaflet
Rare (may affect up to 1 in 10,000 people):
allergic reactions (such as rash and itching).
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products; Al. Jerozolimskie 181C, 02-222 Warsaw, phone:
+ 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Azelastin COMOD
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton (mm/yyyy). The expiry date refers to the last day of that month.
Shelf-life after opening the bottle: Properly used Azelastin COMOD eye drops can be used for 12 weeks after first opening.
Azelastin COMOD eye drops can only be used if the packaging is not damaged before first use.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Azelastin COMOD contains
- The active substance is azelastine hydrochloride at a concentration of 0.05% (0.50 mg/ml). One drop of the medicine with a volume of approximately 30 µl contains 0.015 mg of azelastine hydrochloride.
- The other ingredients are: disodium edetate, hypromellose, sorbitol, sodium hydroxide, solution (for pH adjustment), and water for injections.
What Azelastin COMOD looks like and contents of the pack
Azelastin COMOD is a clear, colorless solution in a 10 ml container with a dropper made of LDPE, with a screw cap made of HDPE and a tamper-evident closure, in a cardboard box.
Each pack contains 1 multidose container.
Edition 07/2024
- 1.3.1 SPC, labelling and package leaflet
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
URSAPHARM Poland sp. z o.o.,
ul. Wybrzeże Gdyńskie 27,
01-531 Warsaw, Poland
Phone: 22 732 07 90
Fax: 22 732 07 99
e-mail: [email protected]
Manufacturer:
URSAPHARM Arzneimittel GmbH,
Industriestraße 35,
66129 Saarbrücken,
Germany
Date of revision of the leaflet:
Edition 07/2024
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterUrsapharm Arzneimittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Azelastin ComodDosage form: Drops, 0.5 mg/mlActive substance: azelastinePrescription not requiredDosage form: Drops, 0.5 mg/mlActive substance: azelastineManufacturer: Farma Mediterrania, S.L. NOVOCAT FARMA S.A.Prescription requiredDosage form: Drops, 0.5 mg/mlActive substance: azelastineManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription not required
Alternatives to Azelastin Comod in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Azelastin Comod in Испания
Online doctors for Azelastin Comod
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Azelastin Comod – subject to medical assessment and local rules.