
Azel-drop Alergia

Ask a doctor about a prescription for Azel-drop Alergia

How to use Azel-drop Alergia
PATIENT INFORMATION LEAFLET: USER INFORMATION
Azel-Drop Allergy, 0.5 mg/ml, eye drops, solution
Azelastine hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this patient information leaflet or as directed by your doctor, pharmacist, or nurse.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, ask your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
- If after 2 days there is no improvement or you feel worse, contact your doctor.
Table of contents of the leaflet:
- 1. What is Azel-Drop Allergy and what is it used for
- 2. Important information before using Azel-Drop Allergy
- 3. How to use the medicine Azel-Drop Allergy
- 4. Possible side effects
- 5. How to store the medicine Azel-Drop Allergy
- 6. Contents of the pack and other information
1. What is the medicine Azel-Drop Allergy and what is it used for
Azel-Drop Allergy contains azelastine, which belongs to a group of antiallergic and antihistaminic medicines.
The medicine Azel-Drop Allergy can be used to treat or prevent symptoms of seasonal, allergic eye disorders (seasonal, allergic conjunctivitis accompanying hay fever) in adults and children over 4 years old.
The medicine Azel-Drop Allergy can also be used to treat symptoms of non-seasonal (year-round) allergic eye disorders (year-round, allergic conjunctivitis) in adults and adolescents over 12 years old.
The medicine Azel-Drop Allergy is not intended for the treatment of eye infections.
You should consult a doctor if there is no improvement or if you feel worse after 2 days of using the medicine.
How the medicine Azel-Drop Allergy works
The medicine Azel-Drop Allergy causes rapid relief of symptoms such as burning, itching, tearing, without causing drowsiness.
Allergy is caused by an excessive response of the body's immune system to external substances. Allergy is triggered by contact with pollen, house dust mites, mold, or pet hair.
The medicine Azel-Drop Allergy inhibits the action of histamine and other inflammatory substances released in larger amounts in people with allergies. In this way, the medicine Azel-Drop Allergy counteracts the symptoms of conjunctivitis.
2. Important information before using the medicine Azel-Drop Allergy
When not to use the medicine Azel-Drop Allergy
If you are allergic to azelastine hydrochloride, benzalkonium chloride, or any of the other ingredients of this medicine (listed in section 6.1).
Warnings and precautions
Before starting to use the medicine Azel-Drop Allergy, consult your doctor or pharmacist:
- if you are not sure if the eye disorder is caused by an allergy. This is especially true in situations where the problem affects only one eye; there is a worsening of vision, eye pain, but no nasal symptoms. This may indicate that the cause is an infection, not an allergy
- if symptoms worsen or persist for more than 48 hours without significant improvement, despite using the medicine Azel-Drop Allergy.
People with conjunctivitis should not wear contact lenses.
Children and adolescents
The medicine Azel-Drop Allergy should be used to treat seasonal allergic disorders in children from 4 years old and to treat non-seasonal (year-round) allergic disorders in adolescents from 12 years old.
Azel-Drop Allergy and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as any medicines you plan to take.
The effect of other medicines, food, or drink on the medicine Azel-Drop Allergy is not known.
Pregnancy and breastfeeding
In pregnancy and during breastfeeding, or if you think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The medicine Azel-Drop Allergy passes into breast milk in small amounts. Therefore, it is not recommended to use the medicine Azel-Drop Allergy during breastfeeding.
Driving and using machines
Mild, transient eye irritation caused by the administration of the medicine may occur. It is unlikely that the medicine Azel-Drop Allergy will significantly impair vision. If you experience any transient vision disturbance, do not drive or operate machinery until your vision returns to normal.
The medicine Azel-Drop Allergy contains benzalkonium chloride
This medicine contains 0.0035 mg of benzalkonium chloride in each drop, which corresponds to a concentration of 0.125 mg/ml.
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. You should remove contact lenses before administration and wait at least 15 minutes before reinserting them.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer on the front of the eye). If you experience any abnormal sensations in the eye, stinging, or eye pain after using the medicine, consult your doctor.
3. How to use the medicine Azel-Drop Allergy
This medicine should always be used exactly as described in this patient information leaflet or as directed by your doctor or pharmacist.
If you need advice or further information, ask your doctor or pharmacist.
Seasonal, allergic conjunctivitis
Unless your doctor has told you otherwise, the usual dose for adults and children over 4 years old is one drop into each eye, twice a day (morning and evening). If necessary, the dose can be increased to one drop, administered 4 times a day.
The medicine Azel-Drop Allergy can be administered prophylactically before expected exposure to the allergen.
Non-seasonal (year-round), allergic conjunctivitis
Unless your doctor has told you otherwise, the usual dose for adults and adolescents over 12 years old is one drop into each eye, twice a day (morning and evening). If necessary, the dose can be increased to one drop, administered 4 times a day.
Method of administration
Remove the cap. Tilt your head slightly and gently pull down the lower eyelid of the eye (Figure 1).
Carefully put 1 drop into the inner part of the lower eyelid (Figure 2). Be careful not to touch the tip of the dropper to the eye or skin. Release the lower eyelid and gently press the corner of the eye towards the nose (Figure 3). Slowly blink several times to spread the drop over the surface of the eyeball. To administer the medicine to the other eye, repeat the above steps.
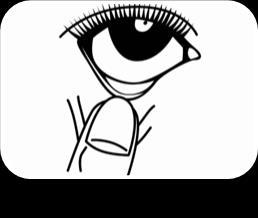
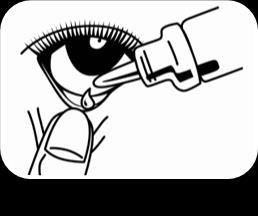
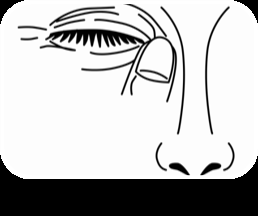
Figure 1 Figure 2 Figure 3
Duration of treatment
Continue using the medicine Azel-Drop Allergy after the symptoms have disappeared, as long as you are exposed to the allergen (e.g., pollen, dust).
Do not use the medicine Azel-Drop Allergy for more than 6 weeks.
If you feel that the effect of the medicine Azel-Drop Allergy is too strong or too weak, tell your doctor or pharmacist.
Using a higher dose of the medicine Azel-Drop Allergy than recommended
After administering the medicine to the eyes, no specific overdose reactions are known and are not expected with this route of administration. If you accidentally swallow the medicine Azel-Drop Allergy, consult your doctor or the nearest hospital emergency department as soon as possible.
Missing a dose of the medicine Azel-Drop Allergy
No special action is necessary if you miss a dose of the medicine Azel-Drop Allergy. Take the next dose at the usual time. If necessary, the medicine can also be administered between two planned administrations.
Stopping the use of the medicine Azel-Drop Allergy
Use the medicine Azel-Drop Allergy regularly until the symptoms disappear, if possible.
If you stop using the medicine Azel-Drop Allergy, there is a risk of recurrence of typical disease symptoms.
If you have any further doubts about the use of the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, the medicine Azel-Drop Allergy can cause side effects, although not everybody gets them.
Possible side effects are listed below by frequency of occurrence.
Common: (may affect up to 1 in 10 people)
Mild, transient eye irritation (e.g., burning, itching, tearing) occurred after administration of the medicine Azel-Drop Allergy.
Uncommon: (may affect up to 1 in 100 people)
Bitter taste in the mouth.
Very rare: (may affect up to 1 in 10,000 people)
Allergic reactions (such as rash and itching).
What to do if you experience side effects
Usually, the listed side effects disappear quickly. No special measures are necessary.
To counteract the bitter taste in the mouth after using the medicine Azel-Drop Allergy, you can drink non-alcoholic beverages (juices, water).
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of the medicine.
5. How to store the medicine Azel-Drop Allergy
Store out of sight and reach of children.
Do not use the medicine Azel-Drop Allergy after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C. Store the bottle in the outer carton to protect from light.
After first opening: do not use the medicine after 4 weeks of first opening the bottle.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What the medicine Azel-Drop Allergy contains
- The active substance is azelastine hydrochloride. 1 ml contains 0.5 mg of azelastine hydrochloride. One drop contains 0.015 mg of azelastine hydrochloride.
- The other ingredients are: benzalkonium chloride; liquid sorbitol 70% (non-crystallizing); hypromellose; disodium edetate; sodium hydroxide (to adjust pH); water for injections.
What the medicine Azel-Drop Allergy looks like and contents of the pack
The medicine Azel-Drop Allergy is a clear, colorless solution. The medicine Azel-Drop Allergy is available in a LDPE bottle with a LDPE dropper and an HDPE cap. One bottle contains 6 ml of solution.
Marketing authorization holder and manufacturer
Marketing authorization holder
Bausch+Lomb Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer
Dr. Gerhard Mann chem.-pharm. Fabrik GmbH
Brunsbütteler Damm 165/173
13581 Berlin
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Member State Name of the medicinal product
Austria
Vividrin Azelastin 0,5 mg/ml Augentropfen, Lösung
Germany
Vividrin Azelastin 0,5 mg/ml Augentropfen, Lösung
Poland
Azel-Drop Allergy
Slovenia
Alezaxin
Italy
Vividrin
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterDr. Gerhard Mann Chem. - Pharm. Fabrik GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Azel-drop AlergiaDosage form: Drops, 0.5 mg/mlActive substance: azelastinePrescription not requiredDosage form: Drops, 0.5 mg/mlActive substance: azelastineManufacturer: Farma Mediterrania, S.L. NOVOCAT FARMA S.A.Prescription requiredDosage form: Drops, 0.5 mg/mlActive substance: azelastineManufacturer: mibe GmbH ArzneimittelPrescription not required
Alternatives to Azel-drop Alergia in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Azel-drop Alergia in Spain
Online doctors for Azel-drop Alergia
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Azel-drop Alergia – subject to medical assessment and local rules.














