
How to use Azelamed
Package Leaflet: Information for the Patient
Azelamed, 0.5 mg/ml, eye drops, solution
Azelastine hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including those not listed in the leaflet, tell your doctor or pharmacist. See section 4.
- If after 2 days there is no improvement or you feel worse, contact your doctor. Relief of allergic conjunctivitis symptoms should occur within 15-30 minutes.
Table of Contents of the Leaflet
- 1. What is Azelamed and what is it used for
- 2. Important information before using Azelamed
- 3. How to use Azelamed
- 4. Possible side effects
- 5. How to store Azelamed
- 6. Contents of the pack and other information
1. What is Azelamed and what is it used for
Azelamed contains azelastine, which belongs to a group of antihistamine medicines. Antihistamines work by preventing the action of histamine, a substance produced by the body as part of an allergic reaction. Azelastine has been shown to reduce eye inflammation. Azelamed can be used to treat or prevent symptoms of seasonal, allergic eye disorders accompanying hay fever (seasonal allergic conjunctivitis) in adults, adolescents, and children aged 4 and above. Azelamed can be used in eye disorders caused by allergy to substances such as house dust mites or animal hair (perennial allergic conjunctivitis) in adults and adolescents aged 12 and above.
Azelamed is not intended for the treatment of eye infections.
If after 2 days there is no improvement or you feel worse, contact your doctor.
2. Important information before using Azelamed
When not to use Azelamed
- If you are allergic to azelastine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Azelamed, discuss it with your doctor or pharmacist. Be particularly careful when using Azelamed:
- if you are not sure whether your eye disorder is caused by an allergy. This is especially true in situations where the problem affects only one eye; there is a worsening of vision or eye pain, but no nasal symptoms. This may indicate that the cause is an infection, not an allergy;
- if symptoms worsen or persist for more than 2 days without significant improvement despite using Azelamed. In such cases, consult your doctor or pharmacist.
Before using Azelamed, contact your doctor, pharmacist, or optician if:
- you wear contact lenses.
Children and adolescents
Treatment of eye disorders accompanying hay fever (seasonal allergic conjunctivitis): Do not use this medicine in children under 4 years of age, as safety and efficacy have not been established. Treatment of eye disorders caused by allergy (perennial allergic conjunctivitis): Do not use this medicine in children under 12 years of age, as safety and efficacy have not been established.
Other medicines and Azelamed
Tell your doctor or pharmacist about all medicines you are taking now or have recently taken, as well as any medicines you plan to take. The effect of other medicines on Azelamed is not known.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
Driving and using machines
After using Azelamed, there may be temporary blurred vision. If this happens, wait until your vision returns to normal before driving or operating machinery.
Azelamed contains benzalkonium chloride
The medicine contains 3.75 micrograms of benzalkonium chloride per drop, equivalent to 0.125 mg/ml. Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Remove contact lenses before administering the drops and wait at least 15 minutes before putting them back. Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or disorders affecting the cornea (the transparent layer on the front of the eye). If you experience any abnormal sensations in the eye, stinging, or eye pain after using the medicine, contact your doctor.
3. How to use Azelamed
Always use this medicine exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist. If you are unsure, consult your doctor or pharmacist. Do not use this medicine if the tamper-evident seal on the bottle neck is broken before the first use. To open the bottle, unscrew the cap by turning it until the seal breaks. Eye disorders caused by hay fever (seasonal allergic conjunctivitis)
- In adults, adolescents, and children aged 4 and above.
- The recommended dose is 1 drop into each eye, morning and evening.
In case of anticipated exposure to an allergen, the recommended dose should be used preventively before leaving the house. Eye disorders caused by allergy (perennial allergic conjunctivitis)
- In adults, adolescents, and children aged 12 and above.
- The recommended dose is 1 drop into each eye, morning and evening.
In case of severe symptoms, your doctor may increase the dose to 1 drop into each eye, four times a day. Relief of allergic conjunctivitis symptoms should occur within 15-30 minutes. If possible, use Azelamed regularly until symptoms resolve. If you stop using Azelamed, there is a risk of recurrence of disease symptoms. Remember:
- not to use Azelamed for longer than 6 weeks,
- to use Azelamed only for the eyes.
Method of administration of Azelamed
To correctly administer the eye drops, you can sit in front of a mirror during the first few administrations.
- 1. Wash your hands.
- 2. Gently dry the area around the eye with a clean, disposable tissue (Fig. 1).
- 3. Unscrew the cap and check that the dropper is clean.
- 4. Gently pull down the lower eyelid (Fig. 2).
- 5. Carefully place a drop inside the lower eyelid (Fig. 3). Be careful not to touch the eye with the dropper.
- 6. Then release the eyelid and gently press the inner corner of the eye from the side of the nose (Fig. 4). Pressing with your finger, perform a few gentle blinks to distribute the medicine evenly over the surface of the eye.
- 7. Remove excess medicine with a clean tissue.
- 8. Repeat the above steps to administer the medicine to the other eye.
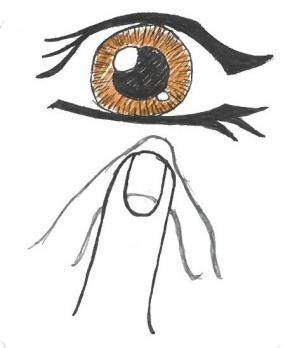
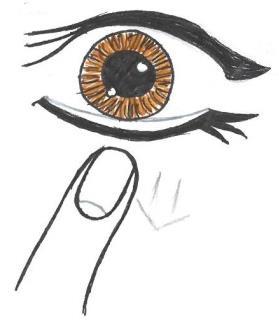
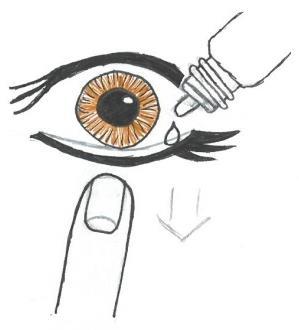
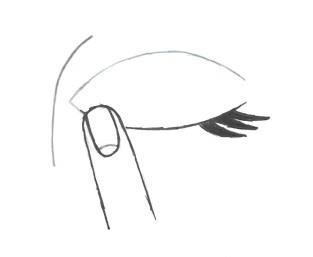
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Using a higher dose of Azelamed than recommended
In case of using too much Azelamed in the eye, it is unlikely to cause adverse effects. If you are unsure, consult your doctor. If you accidentally swallow Azelamed, contact your doctor or the nearest emergency department as soon as possible. There is no experience with toxic (very high, poisonous) doses of azelastine hydrochloride in humans. Based on animal studies, after significant overdose or in case of poisoning, central nervous system disorders (such as restlessness, excitement, or deep, prolonged drowsiness or sleepiness) can be expected. In such cases, symptomatic treatment should be used.
Missing a dose of Azelamed
Do not use a double dose to make up for a missed dose. Take the next dose as soon as possible and then take the medicine at the usual time.
Stopping the use of Azelamed
If you stop using Azelamed, there is a risk of recurrence of disease symptoms. If you have any doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Azelamed can cause side effects, although not everybody gets them. Common (may affect up to 1 in 10 people): Mild, transient irritation (burning, itching, tearing) of the eyes after administration of Azelamed. Uncommon (may affect up to 1 in 100 people): Bitter taste in the mouth. It should pass quickly, especially after drinking a non-alcoholic beverage. Rare (may affect up to 1 in 10,000 people): Allergic reaction (such as rash and itching).
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, e-mail: [email protected]. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Azelamed
Keep out of sight and reach of children. Do not use this medicine after the expiry date stated on the label and carton after "EXP". The expiry date refers to the last day of the month. Do not store in the refrigerator or freeze.
Shelf life after opening the package
Shelf life after first opening the bottle: 6 weeks. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Azelamed contains:
- The active substance is azelastine hydrochloride. 1 ml of solution contains 0.5 mg of azelastine hydrochloride. Each drop contains 0.015 mg of azelastine hydrochloride.
- The other ingredients are: benzalkonium chloride, disodium edetate, hypromellose 4000, sorbitol liquid, crystallizing, sodium hydroxide to adjust pH, and water for injections.
What Azelamed looks like and contents of the pack
Azelamed is a clear, colorless to light yellow solution. Azelamed is available in a bottle with a dropper and a tamper-evident cap. One bottle of 10 ml contains 6 ml of eye drops, solution.
Marketing authorization holder and manufacturer:
Marketing authorization holder
SUN-FARM Sp. z o.o., ul. Dolna 21, 05-092 Łomianki
Manufacturer
mibe GmbH Arzneimittel, Münchener Straße 15, 06796 Brehna, Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Azedil 0.5 mg/ml Augentropfen
Croatia: Azelamed 0.5 mg/ml eye drops, solution
Germany: Azedil 0.5 mg/ml Augentropfen, Lösung
Poland: Azelamed
Date of last revision of the leaflet:05.2018
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- Importermibe GmbH Arzneimittel
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AzelamedDosage form: Drops, 0.5 mg/mlActive substance: azelastinePrescription not requiredDosage form: Drops, 0.5 mg/mlActive substance: azelastineManufacturer: Farma Mediterrania, S.L. NOVOCAT FARMA S.A.Prescription requiredDosage form: Drops, 0.5 mg/mlActive substance: azelastineManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription not required
Alternatives to Azelamed in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Azelamed in Spain
Online doctors for Azelamed
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Azelamed – subject to medical assessment and local rules.







